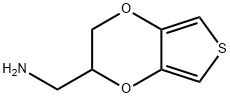
Thieno[3,4-b]-1,4-dioxin-2-methanamine, 2,3-dihydro- synthesis
- Product Name:Thieno[3,4-b]-1,4-dioxin-2-methanamine, 2,3-dihydro-
- CAS Number:1003863-36-2
- Molecular formula:C7H9NO2S
- Molecular Weight:171.22
![2-(Azidomethyl)-2,3-dihydrothieno[3,4-b][1,4]dioxine](/CAS/20211123/GIF/1003863-35-1.gif)
1003863-35-1
4 suppliers
inquiry
![Thieno[3,4-b]-1,4-dioxin-2-methanamine, 2,3-dihydro-](/CAS/20150408/GIF/1003863-36-2.gif)
1003863-36-2
13 suppliers
inquiry
Yield:1003863-36-2 83%
Reaction Conditions:
with triphenylphosphine;sodium hydroxide in tetrahydrofuran;water at 50; for 3 h;
Steps:
3.3C Step3C: Synthesis of 2,-Aminomethyl-3.4-ethylenedioxythiophene fEDOT-
Into a 50 mL two necked round-bottom flask equipped with a reflux condenser, a magnetic stirring bar were charged tetrahydrofuran (10 mL), EDOT-N3 (1.0 g, 5.10 mmol), triphenylphosphine (1.6 g, 6.1 mmol) and 2 mol L- 1 sodium hydroxide aqueous solution (10 mL). The reaction mixture was stirred at 50 °C for 3 h. The reaction mixture was allowed to cool to room temperature. After evaporation of tetrahydrofuran, 2 M hydrochloric acid solution was used to control the pH below 3. Then the aqueous layer was extracted with dichloromethane (2 x 20 mL) and the combined organic layers were discarded. 1 M sodium hydroxide solution was added to adjust the pH of aqueous layer to 12. The aqueous layer was extracted with dichloromethane (3 x 20 mL) and the combined organic layers were dried with anhydrous sodium sulfate. The solvent was removed under reduced pressure to afford EDOT-NH2 as colorless oil (0.72 g, 83 %). XH NMR (400 MHz, CDCb) : d=6.33 (dd, 2H), 4.20 (dd, 1H), 4.12 (m, 1H), 4.00 (dd, 1H), 2.97 (m, 2H), 1.32 (s, 2H) 13C NMR: d = 141.6, 140.5, 99.5, 75.2, 66.6, 42.3 ppm.
References:
WO2021/41874,2021,A1 Location in patent:Page/Page column 24; 25