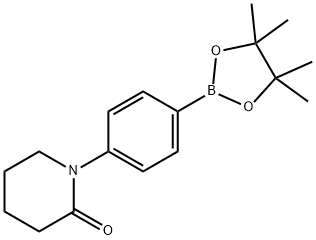
1-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)piperidin-2-one synthesis
- Product Name:1-(4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)piperidin-2-one
- CAS Number:1007346-42-0
- Molecular formula:C17H24BNO3
- Molecular Weight:301.19
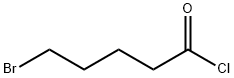
4509-90-4
244 suppliers
$15.00/1g
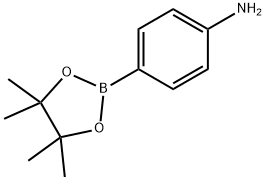
214360-73-3
338 suppliers
$8.00/1g

1007346-42-0
11 suppliers
inquiry
Yield:1007346-42-0 36.1%
Reaction Conditions:
Stage #1: 5-bromovaleroyl chloride;4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)anilinewith triethylamine in dichloromethane at 0 - 20; for 18.5 h;
Stage #2: with sodium hydride in N,N-dimethyl-formamide;mineral oil at 0 - 20; for 21 h;
Steps:
95.1
Step 1: Preparation of 1-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]piperidin-2-one Et3N (239 μL, 1.71 mmol) and 5-bromovaleroyl chloride (166 μL, 1.26 mmol) were added to a solution of 4-aminophenylboronic acid pinacol ester (250 mg, 1.14 mmol) in DCM (5 mL) at 0° C. After stirring the mixture for 18.5 hours at room temperature, the mixture was concentrated. The residue was diluted with EtOAc and washed with sat. NaHCO3 and brine. The organic layer was dried over anhydrous Na2SO4, and concentrated. The residue was diluted with DMF (5 mL) and NaH (55% in liquid paraffin, 62.2 mg, 1.43 mmol) at 0° C. After stirring the mixture for 21 hours at room temperature, the mixture was concentrated. The residue was diluted with 10% aqueous citric acid and extracted with DCM (3*). The combined organic layers were dried over anhydrous Na2SO4, and concentrated. The residue was purified by PTLC (EtOAc/hexane=1:1) to afford desired product (124 mg, 36.1%) as white solid. 1HNMR (CDCl3) 400 MHz δ: 7.86-7.81 (m, 2H), 7.30-7.24 (m, 2H), 3.70-3.60 (m, 2H), 2.61-2.51 (m, 2H), 2.00-1.87 (m, 4H), 1.33 (s, 12H).
References:
US2012/108574,2012,A1 Location in patent:Page/Page column 138