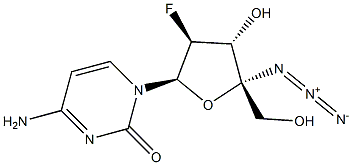
4'-C-azido-2'-deoxy-2'-fluoro-beta-D-arabinocytidine synthesis
- Product Name:4'-C-azido-2'-deoxy-2'-fluoro-beta-D-arabinocytidine
- CAS Number:1011529-10-4
- Molecular formula:C9H11FN6O4
- Molecular Weight:286.2198

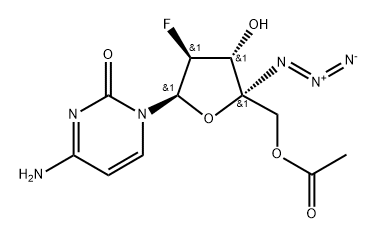
1030595-44-8
1 suppliers
inquiry

1011529-10-4
67 suppliers
inquiry
Yield:1011529-10-4 89%
Reaction Conditions:
with methanol;triethylamine at 20; for 12 h;
Steps:
1
The synthesis of compound xv: The compound xiv (5mmol) was dissolved in 5% methanol-triethylamine (100ml), and the solution was stirred at a room temperature for 12 hours. After completion of the reaction, the solvent was removed through evaporation and the residue was purified by column chromatography to obtain the compound xv (89.0%).ESI-MS: 287[M+H]. 1H NMR(DMSO-d6, 300 MHz)δ: 8.14 (d, 1H), 7.32 (d, 1H), 7.21 (br, 2H), 6.08 (dd, 1H), 5.82 (d, 1H), 5.11 (t, 1H), 4.92 (dt, 1H), 4.16 (dt, 1H), 3.62-3.69 (m, 2H).
References:
EP2177527,2010,A1 Location in patent:Page/Page column 12
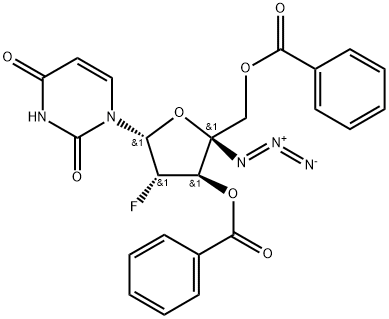
1145869-37-9
6 suppliers
inquiry

1011529-10-4
67 suppliers
inquiry
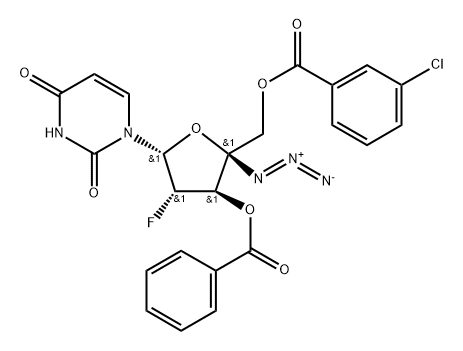
1333126-30-9
24 suppliers
inquiry

1011529-10-4
67 suppliers
inquiry