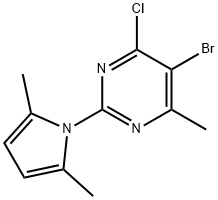
PYRIMIDINE, 5-BROMO-4-CHLORO-2-(2,5-DIMETHYL-1H-PYRROL-1-YL)-6-METHYL- synthesis
- Product Name:PYRIMIDINE, 5-BROMO-4-CHLORO-2-(2,5-DIMETHYL-1H-PYRROL-1-YL)-6-METHYL-
- CAS Number:1013099-50-7
- Molecular formula:C11H11BrClN3
- Molecular Weight:300.58
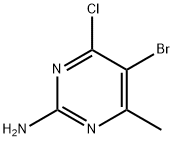
6314-12-1
64 suppliers
$80.00/250mg
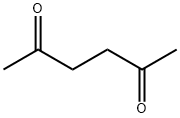
110-13-4
398 suppliers
$10.00/5g

1013099-50-7
17 suppliers
$295.00/100mg
Yield:1013099-50-7 100%
Reaction Conditions:
with toluene-4-sulfonic acid in toluene at 160; for 7 h;Dean-Stark;
Steps:
5-bromo-4-chloro-2-(2,5-dimethyl-1H-pyrrol-1-yl)-6-methylpyrimidine (5c)
A mixture of 5b (85 g, 382 mmol), 2,5-hexanedione (65.4 g, 573 mmol) and p-toluenesulfonic acid monohydrate (7.27 g, 38.2 mmol) in toluene (500 mL) in a round bottom flask connected to a Dean-Stark apparatus was refluxed at 160 °C for 7 h. After cooling to rt, the reaction mixture was washed with water and brine, dried over Na2SO4, filtered and concentrated to afford 5c as a brown solid (115 g, 100% yield), which was used directly in the next step without further purification
References:
Lin, Songwen;Wang, Chunyang;Ji, Ming;Wu, Deyu;Lv, Yuanhao;Sheng, Li;Han, Fangbin;Dong, Yi;Zhang, Kehui;Yang, Yakun;Li, Yan;Chen, Xiaoguang;Xu, Heng [Bioorganic and Medicinal Chemistry,2018,vol. 26,# 3,p. 637 - 646] Location in patent:supporting information
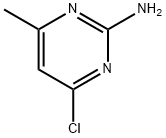
5600-21-5
325 suppliers
$18.00/25g

1013099-50-7
17 suppliers
$295.00/100mg