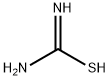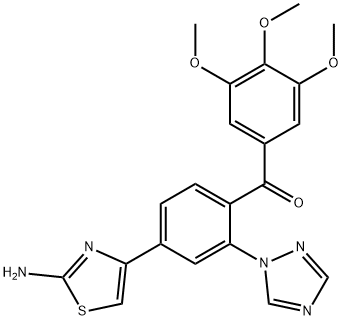
Methanone, [4-?(2-?amino-?4-?thiazolyl)?-?2-?(1H-?1,?2,?4-?triazol-?1-?yl)?phenyl]?(3,?4,?5-?trimethoxyphenyl)?- synthesis
- Product Name:Methanone, [4-?(2-?amino-?4-?thiazolyl)?-?2-?(1H-?1,?2,?4-?triazol-?1-?yl)?phenyl]?(3,?4,?5-?trimethoxyphenyl)?-
- CAS Number:1016543-77-3
- Molecular formula:C21H19N5O4S
- Molecular Weight:437.47
Yield:1016543-77-3 48%
Reaction Conditions:
in ethanol; for 2 h;Heating / reflux;
Steps:
Compound 516; Synthesis of (4-(2-aminothiazol-4-ylV2-( 1 H- 1 ,2.4-triazol- 1 -vPphenvD(3A5-trimethoxyphenyl)methanone; In the same manner as in the synthesis of Compound 515, 1,2,4-triazole was added to Compound 21 of Reaction 6. Then, tributyl(l-ethoxyvinyl)tin was added to the mixture, and the resulting mixture was reacted to obtain (4-(l- ethoxyvinyl)-2-(lH-l,2,4-triazol-l-yl)phenyl)(3,4,5- trimethoxyphenyl)methanone. Thus obtained (4-(l-ethoxyvinyl)-2-( IH- 1,2,4- triazol-l-yl)phenyl)(3,4,5-trimethoxyphenyl)methanone was dissolved in a mixed solution of tetrahydrofuran and water (1/1), and N-bromosuccinimide (NBS) was added thereto. The mixture was stirred for 3 hours at room temperature. After completion of the reaction, the reaction mixture was extracted with EtOAc. The organic layer was washed with a saturated aqueous Na2SO3 solution, water, and brine. The extracted organic layer was dried over anhydrous MgSO4, and the solid substance was filtered off. The filtrate was vacuum evaporated to remove the solvent. The resulting residue was purified by column chromatography (SiO2, n-Hex/EA=3/l→l/2) to obtain l-(3-(lH-l,2,4-triazol-l-yl)-4-(3,4,5-
References:
WO2008/38955,2008,A1 Location in patent:Page/Page column 106-107
![Ethanone, 2-?bromo-?1-?[3-?(1H-?1,?2,?4-?triazol-?1-?yl)?-?4-?(3,?4,?5-?trimethoxybenzoyl)?phenyl]?-](/CAS/20210111/GIF/1016544-80-1.gif)