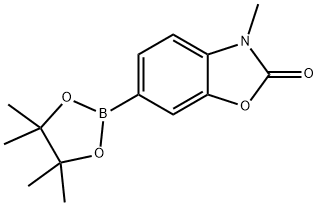
3-Methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]oxazol-2(3H)-one synthesis
- Product Name:3-Methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]oxazol-2(3H)-one
- CAS Number:1016641-53-4
- Molecular formula:C14H18BNO4
- Molecular Weight:275.11
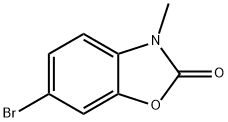
67927-44-0

73183-34-3
![3-Methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]oxazol-2(3H)-one](/CAS/20180702/GIF/1016641-53-4.gif)
1016641-53-4
6-Bromo-3-methylbenzo[d]oxazol-2(3H)-one (1 eq., 17 mmol, 3.8 g), pinacol ester of bisboronic acid (1.1 eq., 18 mmol, 4.7 g), and potassium acetate (2 eq., 33 mmol, 3.3 g) were mixed with trans dichlorobis(triphenylphosphine)palladium(II) (0.03 eq., 0.5 mmol, 0.41 g) in dioxane (180 mL). The reaction mixture was stirred at 100 °C for 7 hours under argon protection. Upon completion of the reaction, water was added and the aqueous layer was extracted with dichloromethane. The organic layers were combined, dried with anhydrous magnesium sulfate and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using dichloromethane as eluent to afford the white powdery product 3-methyl-6-pinacolboronic acid ester benzo[d]oxazol-2(3H)-one (3.7 g, 81% yield, 81% liquid chromatographic purity).

67927-44-0
54 suppliers
$45.00/5mg

73183-34-3
583 suppliers
$6.00/5g
![3-Methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]oxazol-2(3H)-one](/CAS/20180702/GIF/1016641-53-4.gif)
1016641-53-4
32 suppliers
$84.00/100mg
Yield: 81%
Reaction Conditions:
with potassium acetate;trans-bis(triphenylphosphine)palladium dichloride in 1,4-dioxane at 100; for 7 h;
Steps:
3
A mixture of compound B2.2 (1 equiv., 17 mmol, 3.8 g), bis(pinacolato)diboron (1.1 equiv., 18 mmol, 4.7 g), potassium acetate (2 equiv., 33 mmol, 3.3 g) and trans- dichlorobis(triphenylphoshpine) palladium(II) (0.03 equiv., 0.5 mmol, 0.41 g) in dioxane (180 ml) was stirred at 1000C under Ar for 7 h. Water was added and the aqueous layer was extracted with dichloromethane. The organic layer was dried with MgSO4 and concentrated in vacuo. The residue was purified by column chromatography on silica gel (eluens: dichloromethane) to give a white powder B2.3(3.7 g, yield = 81%, purity (LC) = 81%).
References:
TIBOTEC PHARMACEUTICALS LTD. WO2008/37784, 2008, A1 Location in patent:Page/Page column 35; 36
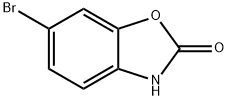
19932-85-5
120 suppliers
$9.00/250mg
![3-Methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]oxazol-2(3H)-one](/CAS/20180702/GIF/1016641-53-4.gif)
1016641-53-4
32 suppliers
$84.00/100mg