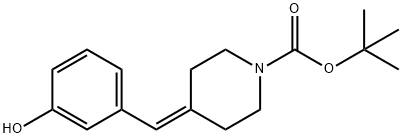
tert-Butyl 4-(3-hydroxybenzylidene)piperidine-1-carboxylate synthesis
- Product Name:tert-Butyl 4-(3-hydroxybenzylidene)piperidine-1-carboxylate
- CAS Number:1020329-87-6
- Molecular formula:C17H23NO3
- Molecular Weight:289.37
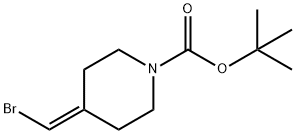
1020329-80-9
64 suppliers
$45.00/10mg
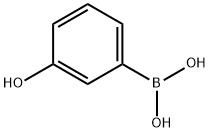
87199-18-6
302 suppliers
$5.00/250mg

1020329-87-6
10 suppliers
inquiry
Yield:1020329-87-6 66%
Reaction Conditions:
with potassium phosphate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in tetrahydrofuran;water at 50; for 1.5 h;
Steps:
57.3
Step 3 tert-Butyl 4-(3-hydroxybenzylidene)piperidine-1-carboxylate To a solution of tert-butyl 4-(bromomethylene)piperidine-1-carboxylate (38 g, 0.1376 mol) in dry THF (380 mL) was added 3-hydroxyphenyl boronic acid (22.77 g, 0.165 mol), potassium phosphate (88.2 g, 0.415 mol) and water (7.6 mL). The mixture was degassed with argon. 1,1'-Bis(diphenylphosphino)ferrocene palladium(II) dichloride dichloromethane complex (11.23 g, 0.01376 mol) was added and the mixture was degassed again. The reaction was heated at 50° C. for 1.5 h and then allowed to cool to RT. Water was added and the mixture was extracted with ethyl acetate (3*). The total organic extract was washed with brine, dried over sodium sulfate and evaporated to dryness. The residue was purified by silica gel chromatography using 100-200 mesh silica gel (8% ethyl acetate in hexane) to give the title compound (26.3 g, 66%). 1H NMR (500 MHz, CDCl3): δ 7.16 (t, J=7.5 Hz, 1H), 6.74 (d, J=7.5 Hz, 1H), 6.68 (d, J=9 Hz, 1H), 6.68 (s, 1H), 6.30 (s, 1H), 5.37 (bs, 1H), 3.49 (m, 2H), 3.40 (m, 2H), 2.46 (m, 2H), 2.31 (m, 2H), 1.48 (s, 9H); 13C NMR (125 MHz, CDCl3): δ 156.09, 155.09, 138.87, 138.15, 129.31, 124.58, 120.98, 115.74, 113.54, 80.10, 45.45, 44.57, 36.08, 29.23, 28.50.
References:
US2008/261941,2008,A1 Location in patent:Page/Page column 37
305794-65-4
15 suppliers
inquiry

1020329-87-6
10 suppliers
inquiry

79099-07-3
0 suppliers
$5.00/5g

1020329-87-6
10 suppliers
inquiry