
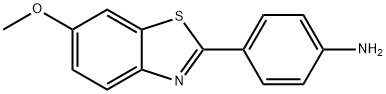
4-(6-Methoxybenzo[d]thiazol-2-yl)-N,N-dimethylaniline synthesis
- Product Name:4-(6-Methoxybenzo[d]thiazol-2-yl)-N,N-dimethylaniline
- CAS Number:10205-71-7
- Molecular formula:C16H16N2OS
- Molecular Weight:284.38
43036-17-5

74-88-4
![4-(6-Methoxybenzo[d]thiazol-2-yl)-N,N-dimethylaniline](/CAS/20200119/GIF/10205-71-7.gif)
10205-71-7

401813-34-1
6-methoxy-2-(4-aminophenyl)benzothiazole (6) (15 mg, 0.059 mmol), methyl iodide (MeI) (8.3 mg, 0.060 mmol) and potassium carbonate (K2CO3) (100 mg, 0.72 mmol) were reacted in anhydrous dimethylsulfoxide (DMSO) (0.5 ml) at 100 °C for 16 hours. The reaction mixture was purified by reversed-phase thin layer chromatography (TLC) (methanol:water=7:1) to afford 6-methoxy-2-(4-methylaminophenyl)benzothiazole (7) (2.0 mg, 13.3%) and 6-methoxy-2-(4-dimethylaminophenyl)benzothiazole (8) (6 mg, 40%). 1H NMR (300MHz, acetone-d6) δ of compound 7: 7.85 (d, J=8.7Hz, 2H, H-2',6'), 7.75 (dd, J=8.8Hz, J=1.3Hz, 1H, H-4), 7.49 (d, J=2.4Hz, 1H, H-7), 7.01 (dd, J=8.8Hz, J=2.4 Hz, 1H, H-5), 6.78 (d, J=7.6 Hz, 2H, H-3',5'), 3.84 (s, 3H, OMe), 2.91 (s, 3H, NMe). 1H NMR (300MHz, acetone-d6) δ of compound 8: 7.85 (d, J=8.7Hz, 2H, H-2',6'), 7.75 (dd, J=8.8Hz, J=1.3Hz, 1H, H-4), 7.49 (d, J=2.4Hz, 1H, H-7), 7.01 (dd, J=8.8Hz, J=2.4 Hz, 1H, H-5), 6.78 (d, J=7.6 Hz, 2H, H-3',5'), 3.84 (s, 3H, OMe), 3.01 (s, 6H, NMe2). Using the same strategy, other 2-(4'-aminophenyl)benzothiazole derivatives can be synthesized by selecting appropriate substituted aniline derivatives (e.g., 2-, 3- or 4-methylaniline) and appropriate 4-nitrobenzoyl chloride derivatives (e.g., 2- or 3-methyl-4-nitrobenzoyl chloride).

82020-41-5
0 suppliers
inquiry
![4-(6-Methoxybenzo[d]thiazol-2-yl)-N,N-dimethylaniline](/CAS/20200119/GIF/10205-71-7.gif)
10205-71-7
22 suppliers
inquiry
Yield:10205-71-7 91%
Reaction Conditions:
with 2,3-dicyano-5,6-dichloro-p-benzoquinone in dichloromethane at 20; for 0.333333 h;
References:
Bose, D. Subhas;Idrees;Srikanth, Bingi [Synthesis,2007,# 6,p. 819 - 823]
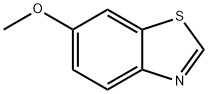
2942-13-4
122 suppliers
$8.00/100mg
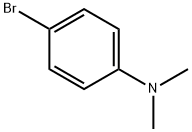
586-77-6
438 suppliers
$5.00/10g
![4-(6-Methoxybenzo[d]thiazol-2-yl)-N,N-dimethylaniline](/CAS/20200119/GIF/10205-71-7.gif)
10205-71-7
22 suppliers
inquiry

43036-17-5
11 suppliers
inquiry

74-88-4
357 suppliers
$15.00/10g
![4-(6-Methoxybenzo[d]thiazol-2-yl)-N,N-dimethylaniline](/CAS/20200119/GIF/10205-71-7.gif)
10205-71-7
22 suppliers
inquiry

401813-34-1
10 suppliers
$282.75/1g

43036-17-5
11 suppliers
inquiry
![4-(6-Methoxybenzo[d]thiazol-2-yl)-N,N-dimethylaniline](/CAS/20200119/GIF/10205-71-7.gif)
10205-71-7
22 suppliers
inquiry