
5,5,8,8-tetramethyl-2,3,5,6,7,8-hexahydro-1H-cyclopenta[b]naphthalen-1-one synthesis
- Product Name:5,5,8,8-tetramethyl-2,3,5,6,7,8-hexahydro-1H-cyclopenta[b]naphthalen-1-one
- CAS Number:102296-82-2
- Molecular formula:C17H22O
- Molecular Weight:242.36
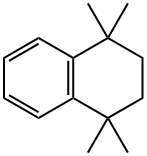
6683-46-1
118 suppliers
$9.00/100mg
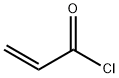
814-68-6
397 suppliers
$21.21/5gm:
![5,5,8,8-tetramethyl-2,3,5,6,7,8-hexahydro-1H-cyclopenta[b]naphthalen-1-one](/CAS/20180702/GIF/102296-82-2.gif)
102296-82-2
14 suppliers
inquiry
Yield:102296-82-2 21.3%
Reaction Conditions:
with aluminum (III) chloride;sodium hydroxide in 1,2-dichloro-benzene at 70; for 7.5 h;Inert atmosphere;Cooling with ice;
Steps:
2 Working Example 2
A polymerization catalyst is prepared similar to Comparative Example 1 but with the catalyst compound of Formula VI.
A new metallocene compound representative of Formula VI is prepared by the following procedure:
Outside of the glovebox, a 500 mL two-necked round bottomed flask equipped with a magnetic stir bar, a powder addition funnel containing aluminum chloride (26.6 g, 199 mmol), a nitrogen inlet, and an outlet which purges to a NaOH scrubber is flushed with dry nitrogen gas.
The vessel is charged with anhydrous o-dichlorobenzene (250 mL) followed by 1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthalene (25.00 g, 132.8 mmol) and acryloyl chloride (12.02 g, 132.8 mmol) and then cooled using an ice water bath.
Aluminum chloride is added over 30 min via the powder addition funnel, then the vessel is warmed to room temperature.
After stirring 1 hr at room temperature, the dark red solution is heated to 70° C. and allowed to react for 6 hours.
The solution is then cooled to room temperature and poured over ice (?300 g) containing conc. HCl (15 mL).
The biphasic mixture is transferred to a separatory funnel; the layers are separated, and the aqueous layer extracted with dichloromethane (300 mL) then ethyl acetate (300 mL).
Combined organic fractions are washed with water (200 mL) and brine (200 mL), dried over anhydrous magnesium sulfate, filtered, and concentrated under vacuum.
Reaction solvent is removed by distillation under high vacuum, leaving a viscous, sticky, red residue.
Hexane is added, and the resultant yellow precipitate collected by filtration and chromatographed (SiO2, hexanes→5% hexanes in EtOAc).
then purified further by vacuum sublimation (100° C., 0.1 torr) to give 5,5,8,8-tetramethyl-2,3,5,6,7,8-hexahydro-1H-cyclopenta[b]naphthalen-1-one as a white solid (6.86 g, 21.3%).
References:
US2019/263944,2019,A1 Location in patent:Paragraph 0133
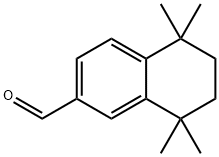
92654-79-0
23 suppliers
$284.00/1g
![5,5,8,8-tetramethyl-2,3,5,6,7,8-hexahydro-1H-cyclopenta[b]naphthalen-1-one](/CAS/20180702/GIF/102296-82-2.gif)
102296-82-2
14 suppliers
inquiry
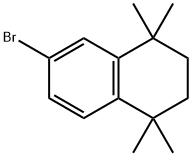
27452-17-1
161 suppliers
$11.00/250mg
![5,5,8,8-tetramethyl-2,3,5,6,7,8-hexahydro-1H-cyclopenta[b]naphthalen-1-one](/CAS/20180702/GIF/102296-82-2.gif)
102296-82-2
14 suppliers
inquiry
![3H-Benz[e]inden-3-one, 1,2,6,7,8,9-hexahydro-6,6,9,9-tetramethyl-](/CAS/20210305/GIF/102296-12-8.gif)
102296-12-8
0 suppliers
inquiry
![5,5,8,8-tetramethyl-2,3,5,6,7,8-hexahydro-1H-cyclopenta[b]naphthalen-1-one](/CAS/20180702/GIF/102296-82-2.gif)
102296-82-2
14 suppliers
inquiry

6683-46-1
118 suppliers
$9.00/100mg
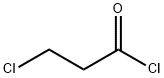
625-36-5
390 suppliers
$45.00/5g
![5,5,8,8-tetramethyl-2,3,5,6,7,8-hexahydro-1H-cyclopenta[b]naphthalen-1-one](/CAS/20180702/GIF/102296-82-2.gif)
102296-82-2
14 suppliers
inquiry