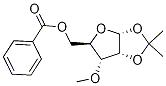
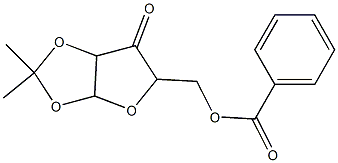
5-O-Benzoyl-1,2-O-isopropylidene-3-O-Methyl-alpha-D-ribofuranose synthesis
- Product Name:5-O-Benzoyl-1,2-O-isopropylidene-3-O-Methyl-alpha-D-ribofuranose
- CAS Number:10300-20-6
- Molecular formula:C16H20O6
- Molecular Weight:308.33
Yield:10300-20-6 95%
Reaction Conditions:
with silver(l) oxide in N,N-dimethyl-formamide at 0 - 20; for 5 h;Inert atmosphere;
Steps:
4.3. Synthesis of 1,2-O-isopropylidene-3-O-methyl-5-O-benzoyl-α-D-ribofuranose (12)
To a solution of (11) (5.0 g, 16.98 mmol) in DMF (50 mL) Ag2O (9.84 g, 42.47 mmol) and CH3I(12.06 g, 84.94 mmol) were added at 0 °C under argon atmosphere. After addition, the reaction mixture was stirred for 5 h at room temperature and then filtered with celite. The filtrated was diluted withEtOAc (300 mL) and washed with distilled water (200 mL 2), sat. NaHCO3 (200 mL 2), brine(200 mL 2), and dried with anhydrous Na2SO4. After filtration, the filtrate was evaporated underreduced pressure and purified by silica gel column to give a white solid (12) (4.98 g, 16.15 mmol, 95%).Rf = 0.3 (PE/ EtOAc = 4:1, V/V); m.p. 74-75 °C; [α]25D + 72.82 (c = 0.103, CH2Cl2); 1H NMR (400 MHz,CDCl3) 8.05 (d, J = 7.6 Hz, 2H), 7.56 (t, J = 7.4 Hz, 1H), 7.43 (t, J = 7.7 Hz, 2H), 5.81 (d, J = 3.5 Hz, 1H),4.71 (t, J = 3.9 Hz, 1H), 4.65 (dd, J = 12.2, 2.2 Hz, 1H), 4.41 (dd, J = 12.2, 5.0 Hz, 1H), 4.33 4.27 (m, 1H),3.63 (dd, J = 9.1, 4.2 Hz, 1H), 3.49 (s, 3H), 1.61 (s, 3H), 1.37 (s, 3H); 13C NMR (100 MHz, CDCl3) 166.5,133.3, 130.2, 130.0 (C 2), 128.6 (C 2), 113.4, 104.4, 81.3, 77.6, 76.7, 63.6, 58.7, 27.0, 26.7; HRESIMS m/z:[M + Na]+ calcd. for C16H20O6Na 331.1152, found 331.1136.
References:
Ding, Haixin;Ruan, Zhizhong;Kou, Peihao;Dong, Xiangyou;Bai, Jiang;Xiao, Qiang [Marine Drugs,2019,vol. 17,# 4,art. no. 226]
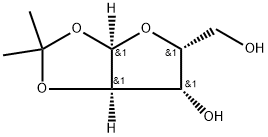
20031-21-4
265 suppliers
$6.00/1g

10300-20-6
12 suppliers
inquiry
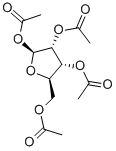
13035-61-5
586 suppliers
$5.00/25g

10300-20-6
12 suppliers
inquiry
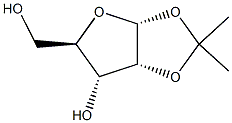
37077-81-9
43 suppliers
inquiry

10300-20-6
12 suppliers
inquiry
6698-46-0
20 suppliers
inquiry

10300-20-6
12 suppliers
inquiry
![(4-hydroxy-7,7-dimethyl-2,6,8-trioxabicyclo[3.3.0]oct-3-yl)methyl benz oate](/CAS/GIF/6612-91-5.gif)
