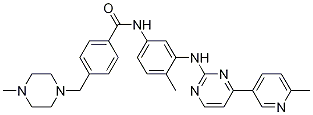
BenzaMide, N-[4-Methyl-3-[[4-(6-Methyl-3-pyridinyl)-2-pyriMidinyl]aMino]phenyl]-4-[(4-Methyl-1-piperazinyl)Methyl]- synthesis
- Product Name:BenzaMide, N-[4-Methyl-3-[[4-(6-Methyl-3-pyridinyl)-2-pyriMidinyl]aMino]phenyl]-4-[(4-Methyl-1-piperazinyl)Methyl]-
- CAS Number:1032314-85-4
- Molecular formula:C30H33N7O
- Molecular Weight:507.6293
![Benzamide, N-[3-[(1,6-dihydro-6-oxo-2-pyrimidinyl)amino]-4-methylphenyl]-4-[(4-methyl-1-piperazinyl)methyl]-](/CAS/20210305/GIF/1451042-78-6.gif)
1451042-78-6
0 suppliers
inquiry
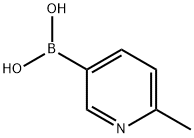
659742-21-9
226 suppliers
$12.00/250mg
![BenzaMide, N-[4-Methyl-3-[[4-(6-Methyl-3-pyridinyl)-2-pyriMidinyl]aMino]phenyl]-4-[(4-Methyl-1-piperazinyl)Methyl]-](/CAS/GIF/1032314-85-4.gif)
1032314-85-4
4 suppliers
inquiry
Yield:1032314-85-4 51%
Reaction Conditions:
Stage #1: 4-[(4-methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[(4-oxo-2-pyrimidinyl)amino]-phenyl]benzamidewith triethylamine;bromo-tris(1-pyrrolidinyl)phosphonium hexafluorophosphate in 1,4-dioxane at 45; for 1.5 h;Inert atmosphere;
Stage #2: (6-methylpyridin-3-yl)boronic acidwith bis-triphenylphosphine-palladium(II) chloride in 1,4-dioxane at 20; for 1 h;Inert atmosphere;
Stage #3: with sodium carbonate in 1,4-dioxane;water; for 24 h;Inert atmosphere;Reflux;
Steps:
6 4-[(4-Methyl-1-piperazinyl)methyl]-N-[4-methyl-3-[[4-(6-methylpyridin-3yl)-2-pyrimidinyl]amino]-phenyl]benzamide (WBZ-4)
To a suspension of 4-[(4-methyl-1 -piperazinyl)methyl]-N-[4-methyl-3-[(4- oxo-2-pyrimidinyl)amino]phenyl]benzamide (0.30 g, 0.69 mmol) in purified dioxane (15 ml) PyBrOP (0.34 g, 0.73 mmol) and triethylamine (0.29 ml, 2.1 mmol) were added under argon. The mixture was stirred at 45 °C for 1.5 h. Then 6-methyl-3-pyridylboronic acid (0.10 g, 0.73 mmol) and Pd(PPh3)2Cl2 (24.3 mg, 5 mol-%) there were added and the reaction was stirred at ambient temperature for 1 h. After aqueous Na2CO3 solution (2.1 ml, 1 mol/L; 2.1 mmol) was transferred into mixture by syringe the reaction was refluxed for 24 h under argon. Reaction mixture diluted with ethyl acetate (30 ml) and brine (5 % solution) was filtered through celite. Organic phase was separated and dried. After evaporation of solvent under reduced pressure the residue was treated with methanol to obtain 0.18 g (51 %) of title compound. Analytical sample could be obtained by flash chromatography (EtOAc:MeOH 10: 1 followed by the eluent with addition of NH4OH). M.p. 202- 205°C 1 H NMR (DMSO-ofe): 2.15 (s,3H), 2.21 (s,3H), 2.25- 2.45 (m, 8H), 2.52 (s,3H), 3.52 (s, 2H), 7.19 (d J= 8.5 Hz, 1 H) 7.37 (d J= 8.5 Hz, 1 H), 7.38 (d J= 5.2 Hz, 1 H), 7.43 (d J= 8.3 Hz, 2H), 7.48 (dd J= 8.2; 2.2 Hz, 1 H), 7.90 (d J= 8.3 Hz, 2H). 8.05 (d J= 2.0 Hz, 1 H), 8.36 (dd J= 8.2; 2.3 Hz, 1 H), 8.47 (d J=5.2 Hz, 1 H), 8.93 (s, 1 H), 9.14 (d J=2.3 Hz), 10.15 (s, 1 H).
References:
WO2013/120852,2013,A1 Location in patent:Page/Page column 15; 16
![4(3H)-Pyrimidinone, 2-[(2-methyl-5-nitrophenyl)amino]-](/CAS/20210305/GIF/1025717-41-2.gif)
1025717-41-2
1 suppliers
inquiry
![BenzaMide, N-[4-Methyl-3-[[4-(6-Methyl-3-pyridinyl)-2-pyriMidinyl]aMino]phenyl]-4-[(4-Methyl-1-piperazinyl)Methyl]-](/CAS/GIF/1032314-85-4.gif)
1032314-85-4
4 suppliers
inquiry
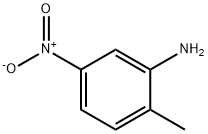
99-55-8
417 suppliers
$6.00/25g
![BenzaMide, N-[4-Methyl-3-[[4-(6-Methyl-3-pyridinyl)-2-pyriMidinyl]aMino]phenyl]-4-[(4-Methyl-1-piperazinyl)Methyl]-](/CAS/GIF/1032314-85-4.gif)
1032314-85-4
4 suppliers
inquiry
![4(3H)-Pyrimidinone, 2-[(5-amino-2-methylphenyl)amino]-](/CAS/20210305/GIF/1451042-77-5.gif)
1451042-77-5
1 suppliers
inquiry
![BenzaMide, N-[4-Methyl-3-[[4-(6-Methyl-3-pyridinyl)-2-pyriMidinyl]aMino]phenyl]-4-[(4-Methyl-1-piperazinyl)Methyl]-](/CAS/GIF/1032314-85-4.gif)
1032314-85-4
4 suppliers
inquiry