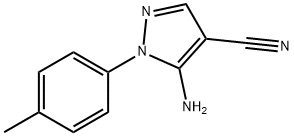
5-AMINO-1-(4-METHYLPHENYL)-1H-PYRAZOLE-4-CARBONITRILE synthesis
- Product Name:5-AMINO-1-(4-METHYLPHENYL)-1H-PYRAZOLE-4-CARBONITRILE
- CAS Number:103646-82-8
- Molecular formula:C11H10N4
- Molecular Weight:198.22
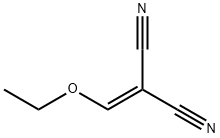
123-06-8
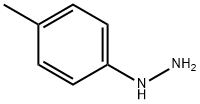
539-44-6

103646-82-8
GENERAL METHOD: p-Methylphenylhydrazine (0.001 mol) was mixed with 10 mL of ethanol, stirred and heated to reflux. Subsequently, ethoxymethylene malononitrile (0.001 mol) dissolved in 10 mL of ethanol was added slowly and dropwise. The reaction mixture was kept at reflux for 2 hours. After completion of the reaction, the mixture was poured into 50 mL of ice water. The precipitated solid product was collected by filtration and washed with cold water to give the final 5-amino-1-toluene-1H-pyrazole-4-carbonitrile in 61-80% yield.

123-06-8
350 suppliers
$14.14/1gm:

539-44-6
77 suppliers
inquiry

103646-82-8
82 suppliers
$32.00/250mg
Yield: 80%
Reaction Conditions:
in ethanol for 2 h;Reflux;
Steps:
General procedure for preparing 5-amino-1-phenyl-1H-pyrazole-4-carbonitriles (10a-c)
General procedure: A mixture of the appropriate phenylhydrazine (0.001 mol)and 10 mL of ethanol was stirred and allowed to reflux.Then, 2-(ethoxymethylene)malononitrile (0.001 mol) dissolvedin 10 mL of ethanol was slowly added. The reactionmixture was refluxed for 2 h. The reaction mixture waspoured into 50 mL of ice-cold water. The precipitate wascollected by filtration and washed with water to provide10a-c in 61-80% yield.
References:
Silveira, Flávia F.;Feitosa, Lívia M.;Mafra, João C. M.;Ferreira, Maria de Lourdes G.;Rogerio, Kamilla R.;Carvalho, Leonardo J. M.;Boechat, Nubia;Pinheiro, Luiz C. S. [Medicinal Chemistry Research,2018,vol. 27,# 8,p. 1876 - 1884]
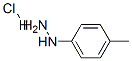
637-60-5
322 suppliers
$6.00/5g

123-06-8
350 suppliers
$14.14/1gm:

103646-82-8
82 suppliers
$32.00/250mg

86240-43-9
1 suppliers
inquiry
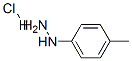
637-60-5
322 suppliers
$6.00/5g

103646-82-8
82 suppliers
$32.00/250mg