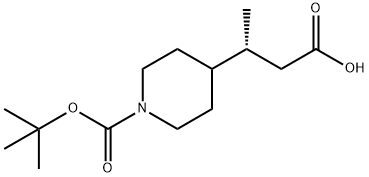
(BETAR)-1-BOC-BETA-METHYL-4-PIPERIDINEPROPANOIC ACID synthesis
- Product Name:(BETAR)-1-BOC-BETA-METHYL-4-PIPERIDINEPROPANOIC ACID
- CAS Number:1037754-69-0
- Molecular formula:C14H25NO4
- Molecular Weight:271.35
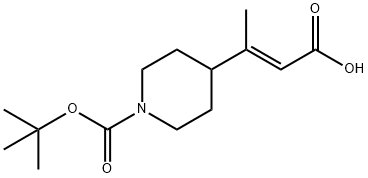
1037754-65-6
7 suppliers
inquiry

1037754-69-0
1 suppliers
inquiry
Yield:98.2 % ee
Reaction Conditions:
with hydrogen;di(norbornadiene)rhodium(I) tetrafluoroborate;(S)-1-[(Rp)-2-(di-tert-butylphosphino)ferrocenyl]ethylbis(2-methylphenyl)phosphine in methanol at 35; under 787.579 Torr; for 48 h;
Steps:
9
Preparation 9: tert-Butyl 4-((R)-2-carboxy-l-methylethyl)piperidine-l-carboxylate. tert-Butyl 4-((E)-2-carboxy-l-methylvinyl)piperidine-l-carboxylate (Preparation 8, 130.0 g, 0.483 mol) was placed in a hydrogenation flask under an Ar atmosphere, then degassed MeOH (400 mL) was added. [Rh(norbornadiene)2]BF4 (1.80 g, 4.81 mmol) and (S)-l-[(R)-2- (di-tert-butylphosphino)ferrocenyl]ethylbis(2-methylphenyl)phosphine (2.90 g, 5.08 mmol) were placed in a separate Schlenk flask under Ar, before being treated with degassed MeOH (200 mL). This catalyst mixture was stirred for 15 min at ambient temperature, before being transferred via cannula into the hydrogenation flask. The Schlenk flask was rinsed with more degassed MeOH (100 mL). These washings were transferred to the hydrogenation flask, then more degassed MeOH (300 mL) was added. The hydrogenation flask was sealed, the Ar replaced by H2, and the pressure set to 1.05 bar. The reaction mixture was heated to 35°C, and stirring/shaking was started. After 48 h, the reaction was stopped and a representative sample of the reaction mixture was analysed by HPLC and 1H NMR. The conversion was 100% and the enantiomeric purity of the crude (R)-acid was 98.2%, as ascertained by the following HPLC method: Column: CHIRALPAK AD-H (previously used with CF3CO2H-containing solvents) 4.6 x 250 mm; Solvent: C6H14-ZPrOH (97:3 isocratic); Temperature: 200C; Flow rate: 1 mL/min; UV-detection (210, 230 nm); Sample: 100 μL reaction solution dissolved with 1 mL MeOH. Retention times: (S)-acid: 19.3 min, (R)-acid: 20.6 min, starting enoic acid: 22.1 min. Isolation procedure: The MeOH was evaporated, then the crude hydrogenation product was dissolved in ^-BuOMe and extracted with aqueous NaOH. The aqueous phase was added to a mixture of IM HCl and EtOAc. The aqueous phase was extracted further with EtOAc, then the combined organic extracts were washed with brine and dried (MgSO4). The title compound was isolated following filtration and complete removal of the solvent.
References:
WO2008/81204,2008,A1 Location in patent:Page/Page column 14-15