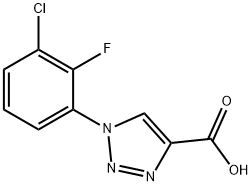
1-(3-chloro-2-fluorophenyl)-1H-1,2,3-triazole-4-carboxylic acid synthesis
- Product Name:1-(3-chloro-2-fluorophenyl)-1H-1,2,3-triazole-4-carboxylic acid
- CAS Number:1039876-00-0
- Molecular formula:C9H5ClFN3O2
- Molecular Weight:241.61
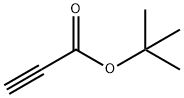
13831-03-3
163 suppliers
$26.00/1g
2106-04-9
257 suppliers
$6.00/10g

1039876-00-0
9 suppliers
inquiry
Yield:-
Reaction Conditions:
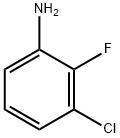
Stage #1: 3-chloro-2-fluoroanilinewith trifluoroacetic acid;sodium nitrite in water at 0; for 0.5 h;
Stage #2: with sodium azide in water at 20;
Stage #3: tert-butyl prop-2-ynoatewith copper diacetate;potassium carbonate;sodium L-ascorbate;L-proline in water;dimethyl sulfoxide at 75;
Steps:
23A 23 A. Preparation of 1 -(3 -chloro-2-fluorophenyl)- 1 H- 1,2,3 -triazole-4-carboxylic acid
To a solution of 3-chloro-2-fluoroaniline (1.2 g, 8.24 mmol) in TFA (4m1), H20 (2m1) was added and the mixture was cooled to 0 °C. NaNO2 (1.12 g, 16.24 mmol) in H20 (2m1) was added dropwise (temperature kept <5°C). After 0.5h, NaN3 (0.5 85 g, 9.0 mmol) was added portionwise. After stirring at rt overnight, the reaction was quenchedwith H20 (lOOml), extracted with EtOAc(2x50m1), dried over Na2SO4, and concentrated to give a solid mass. To a solution of the solid dissolved in DMSO (5 ml), L-proline (0.02g), Cu(OAc)2 (0.lg) K2C03 (1.5g), sodium ascorbate (0.lg), and excess t-butyl propiolate (3 ml) were added and the mixture heated at 75°C overnight. The reaction was quenched with H20 (lOOml), extracted with EtOAc (2 x lOOml), washed with brine(50m1), dried (MgSO4), filtered, and concentrated. The crude product was purifed by normal phase chromatography to give the t-butyl ester intermediate. An aliquot of this material (0.2g) was dissolved in DCM (2m1) and treated with TFA (1 ml) at rt. After stirring overnight, the reaction was quenched with H20 (50m1), extracted with EtOAc (2 xlOOml), dried (MgSO4), filtered, and evaporated to give a brown solid mass. This material was carried forward as is without further purification. ). (ESI) m/z: 342.1 (M+H). 342.1(M+H). ‘HNMR (400MHz, CD3OD/CDC13) ? 8.20(s, 1H), 7.60-7.55(dt, 1H), 7.42-7.37(dt, 1H), 7.28-7.23(dt, 1H).
References:
WO2016/205482,2016,A1 Location in patent:Page/Page column 125; 126

2106-04-9
257 suppliers
$6.00/10g

1039876-00-0
9 suppliers
inquiry

1152597-56-2
4 suppliers
inquiry

1039876-00-0
9 suppliers
inquiry