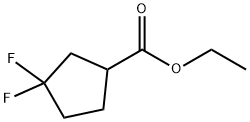
Cyclopentanecarboxylic acid, 3,3-difluoro-, ethyl ester synthesis
- Product Name:Cyclopentanecarboxylic acid, 3,3-difluoro-, ethyl ester
- CAS Number:1042153-25-2
- Molecular formula:C8H12F2O2
- Molecular Weight:178.18
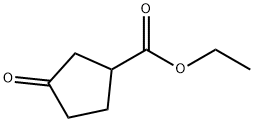
5400-79-3
130 suppliers
$30.00/1g

1042153-25-2
9 suppliers
inquiry
Yield:1042153-25-2 61%
Reaction Conditions:
with diethylamino-sulfur trifluoride in dichloromethane; for 72 h;Reflux;Reagent/catalyst;
Steps:
4 4.4 Ethyl 3,3-difluorocyclopentane carboxylate (25)
To a solution of DAST (45.1g, 37.0mL, 282mmol) in CH2Cl2 (450mL) a solution of 22 (11.0g, 70.4mmol) in CH2Cl2 (150mL) was added at rt. The reaction mixture was refluxed until the starting material had disappeared (ca. 72h, monitored by 1H NMR probe), then cooled, and saturated aq NaHCO3 was added to pH=7. The aqueous phase was extracted with CH2Cl2 (2×100mL), the combined organic extracts were dried over Na2SO4 and evaporated in vacuo. The residue was distilled under reduced pressure. Yield 7.71g, 61%. Colorless oil. Bp 31-32°C/1.8mbar. 1H NMR (CDCl3, 400MHz): δ=4.17 (q, J=7.1Hz, 2H), 3.05-2.93 (m, 1H), 2.47-2.31 (m, 2H), 2.28-1.97 (m, 4H), 1.27 (t, J=7.1Hz, 3H). 13C NMR (CDCl3, 101MHz): δ=173.3, 131.2 (dd, J=250, 246Hz), 60.6, 40.6 (dd, J=5.4, 2.9Hz), 38.1 (t, J=26.6Hz), 34.7 (t, J=25.2Hz), 26.2 (dd, J=4.4, 3.5Hz), 13.9. 19F NMR (CDCl3, 376MHz): δ=-92.0 (dquint, J=229, 16.4Hz), -93.2 (dquint, J=229, 16.4Hz). MS (EI): m/z=178 (M+), 158 (M+-HF). Anal. Calcd for C8H12F2O2: C, 53.93; H, 6.79. Found: C, 53.89; H, 6.39.
References:
Melnykov, Kostiantyn P.;Nosik, Pavel S.;Kurpil, Bohdan B.;Sibgatulin, Dmitriy A.;Volochnyuk, Dmitriy M.;Ryabukhin, Sergey V.;Grygorenko, Oleksandr O. [Journal of Fluorine Chemistry,2017,vol. 199,p. 60 - 66]
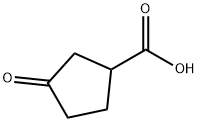
98-78-2
233 suppliers
$10.00/250mg

1042153-25-2
9 suppliers
inquiry