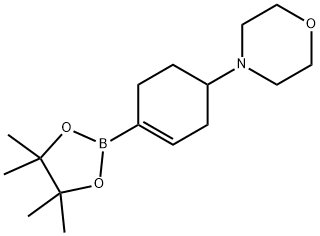
4-[4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-3-CYCLOHEXEN-1-YL]-MORPHOLINE synthesis
- Product Name:4-[4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-3-CYCLOHEXEN-1-YL]-MORPHOLINE
- CAS Number:1046793-56-9
- Molecular formula:C16H28BNO3
- Molecular Weight:293.21
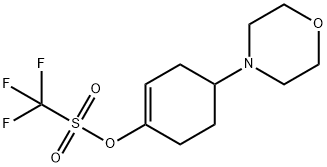
1046793-55-8
4 suppliers
inquiry

73183-34-3
590 suppliers
$6.00/5g
![4-[4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-3-CYCLOHEXEN-1-YL]-MORPHOLINE](/CAS2/GIF/1046793-56-9.gif)
1046793-56-9
48 suppliers
$495.94/5MG
Yield: 86%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane for 12 h;Reflux;Inert atmosphere;
Steps:
2 Step 2: 4-(4-(4,4,5,5-tetramethyl- 1,3,2-dioxaborolan-2-yl)cyclohex-3-enyl)morpholine
[00305] A mixture of 4-morpholinocyclohex-1-enyl trifluoromethanesulfonate (4 g, 12.69 mmol, 1.00 equiv), 4,4,5 ,5-tetramethyl-2- (tetramethyl- 1,3 ,2-dioxaborolan-2-yl)- 1,3,2- dioxaborolane (3.87 g, 15.24 mmol, 1.20 equiv), potassium acetate (3.73 g, 38.01 mmol, 3.00 equiv) and Pd(dppf)C12 (930 mg, 1.27 mmol, 0.10 equiv) in 1,4-dioxane (100 mL) was refluxed under nitrogen for 12 h. The reaction mixture was cooled to room temperature, filtered and then concentrated under vacuum. The residue was purified on a silica gel column eluted with 50-100% of ethyl acetate in petroleum ether to give 3.2 g (86%) of 4-(4-(4,4,5,5- tetramethyl- 1,3 ,2-dioxaborolan-2-yl)cyclohex-3-enyl)morpholine as a yellow oil. ‘H-NMR (300 MHz, CDC13): 6.60-6.55 (m, 1H), 3.80-3.66 (m, 4H), 2.70-2.25 (m, 8H), 2.20-1.90 (m, 4H), 1.25 (s, 12H) ppm.
References:
EPIZYME, INC.;CHESWORTH, Richard;MITCHELL, Lorna, Helen;SHAPIRO, Gideon WO2014/153226, 2014, A1 Location in patent:Paragraph 00305
139025-93-7
66 suppliers
$33.00/100mg
![4-[4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-3-CYCLOHEXEN-1-YL]-MORPHOLINE](/CAS2/GIF/1046793-56-9.gif)
1046793-56-9
48 suppliers
$495.94/5MG
![1,4-Dioxaspiro[4.5]decan-8-one](/CAS/GIF/4746-97-8.gif)
4746-97-8
488 suppliers
$5.00/1g
![4-[4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-3-CYCLOHEXEN-1-YL]-MORPHOLINE](/CAS2/GIF/1046793-56-9.gif)
1046793-56-9
48 suppliers
$495.94/5MG
![4-(1,4-dioxaspiro[4.5]decan-8-yl)morpholine](/CAS2/GIF/127562-53-2.gif)
127562-53-2
22 suppliers
$184.00/250mg
![4-[4-(4,4,5,5-TETRAMETHYL-1,3,2-DIOXABOROLAN-2-YL)-3-CYCLOHEXEN-1-YL]-MORPHOLINE](/CAS2/GIF/1046793-56-9.gif)
1046793-56-9
48 suppliers
$495.94/5MG