
Ethanone, 1-[2-chloro-6-(trifluoroMethyl)-4-pyridinyl]- synthesis
- Product Name:Ethanone, 1-[2-chloro-6-(trifluoroMethyl)-4-pyridinyl]-
- CAS Number:1060810-89-0
- Molecular formula:C8H5ClF3NO
- Molecular Weight:223.58
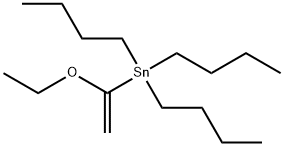
97674-02-7
233 suppliers
$15.00/1g
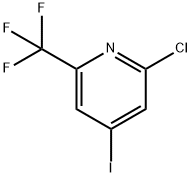
205444-22-0
120 suppliers
$8.00/250mg
![Ethanone, 1-[2-chloro-6-(trifluoroMethyl)-4-pyridinyl]-](/CAS/GIF/1060810-89-0.gif)
1060810-89-0
14 suppliers
inquiry
Yield:1060810-89-0 85 %
Reaction Conditions:
Stage #1: tributyl(1-ethoxyvinyl)stannane;2-chloro-4-iodo-6-(trifluoromethyl)pyridinewith bis-triphenylphosphine-palladium(II) chloride in toluene at 110;Inert atmosphere;
Stage #2: with hydrogenchloride in water;toluene at 20;
Steps:
9 A first intermediate (1-(2-chloro-6-(trifluoromethyl)pyridin-4-yl)ethan-1-one) is made as follows
To a mixture of 2-chloro-4-iodo-6-(trifluoromethyl)pyridine (5.00 g, 16.26 mmol; CAS No.: 205444-22-0) and bis(triphenylphosphine) palladium l)dichloride (570.8 mg, 0.81 mmol) in toluene (90 mL) was added tributyl(1-ethoxyvinyl)stannane (6.6 mL, 19.38 mmol). The reaction mixture was stirred for 16 h at 110 °C under nitrogen protection and then cooled to room temperature. The mixture was added HCI solution (4.0 M aqueous, 20.33 mL, 81.32 mmol) and stirred at room temperature for 3 h. The reaction mixture was diluted with ethyl acetate (200 mL), which was washed with water (2 x 100 mL). The organic layer was separated, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel chromatography (mobile phase: ethyl acetate/petroleum ether, gradient 0% to 40%) to afford 1-(2-chloro-6- (trifluoromethyl)pyridin-4-yl)ethan-1-one (3.10 g, 85% yield) as a yellow oil. LCMS [M+H]+ = 223.7.
References:
WO2022/169998,2022,A1 Location in patent:Paragraph 0024; 0265-0266

1196154-43-4
26 suppliers
inquiry

75-16-1
274 suppliers
$12.00/10ml

1379375-72-0
3 suppliers
inquiry
![Ethanone, 1-[2-chloro-6-(trifluoroMethyl)-4-pyridinyl]-](/CAS/GIF/1060810-89-0.gif)
1060810-89-0
14 suppliers
inquiry