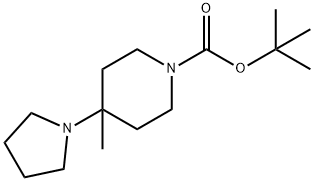
1-Piperidinecarboxylic acid, 4-methyl-4-(1-pyrrolidinyl)-, 1,1-dimethylethyl ester synthesis
- Product Name:1-Piperidinecarboxylic acid, 4-methyl-4-(1-pyrrolidinyl)-, 1,1-dimethylethyl ester
- CAS Number:1061683-26-8
- Molecular formula:C15H28N2O2
- Molecular Weight:268.39

110-52-1
552 suppliers
$9.00/1g
343788-69-2
116 suppliers
$27.00/250mg

1061683-26-8
2 suppliers
inquiry
Yield:1061683-26-8 27%
Reaction Conditions:
with potassium carbonate in acetonitrile at 100; for 18 h;Sealed tube;
Steps:
11.Z Method Z: tert-butyl 4-methyl-4-(pyrrolidin-1-yl)piperidine-1-carboxylate
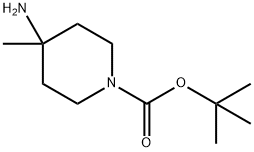
Method Z: tert-butyl 4-methyl-4-(pyrrolidin-1-yl)piperidine-1-carboxylate A mixture of tert-butyl 4-amino-4-methylpiperidine-1-carboxylate (300 mg, 1.4 mmol), potassium carbonate (390 mg, 2.8 mmol) and 1,4-dibromobutane (367 mg, 1.7 mmol) in acetonitrile (10 ml) was placed in a sealed tube and stirred at 100° C. for 18 hours. The solid was filtered off and the filtrate was concentrated in vacuo. The residue was purified on silica gel by flash column chromatography to afford the desired compound (102 mg, 27% yield). 1H NMR (CDCl3, 400 MHz) δ 1.00 (3H, s), 1.38-1.50 (11H, m), 1.69-1.83 (6H, m), 2.60-2.72 (4H, m), 3.33-3.54 (4H, m).
References:
US2010/137305,2010,A1 Location in patent:Page/Page column 37