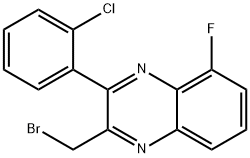
2-(Bromomethyl)-3-(2-chlorophenyl)-5-fluoroquinoxaline synthesis
- Product Name:2-(Bromomethyl)-3-(2-chlorophenyl)-5-fluoroquinoxaline
- CAS Number:1064137-04-7
- Molecular formula:C15H9BrClFN2
- Molecular Weight:351.6

131504-95-5
1 suppliers
inquiry
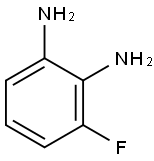
18645-88-0
111 suppliers
$19.00/250mg

1064137-04-7
4 suppliers
inquiry

1064137-02-5
4 suppliers
inquiry
Yield:-
Reaction Conditions:
in ethyl acetate at 20; for 3 h;
Steps:
84
Example 84: Preparation of N-((3-(2-ChlorophenyI)-8-fluoroquinoxalin-2-yl)- methyI)-9H-purin-6-amine as a TFA salt and N-((3-(2-chlorophenyl)-5- fluoroquinoxalin-2-yl)methyl)-9H-purin-6-amine as a TFA salt; 3-(Bromomethyl)-2-(2-chIorophenyl)-5-fluoroquinoxaline and 2- (Bromomethyl)-3-(2-chlorophenyl)-5-fluoroquinoxalineas a mixture over two stepsTo a solution of 3-bromo-l-(2-chlorophenyl)prpane-l,2-dione (Prepared in Example 81, 2.3832 g, 9.114 mmol) in 61 mL of EtOAc was added a solution of 3-fluorobenzene- 1,2 -diamine (1.150 g, 9.114 mmol) at room temperature and the resulting red mixture was stirred at room temperature. After 3 h, the mixture was concentrated in vacuo to give a mixture of two regioisomers as a black syrup. The black syrup was purified by column chromatography on a 80 g of Redi-Sep column using 0 to 50% gradient of EtOAc in hexane over 25 min and then 100% isocratic of EtOAc for 4 min as eluent to give a mixture of 3-(bromomethyl)-2-(2- chlorophenyl)-5-fluoroquinoxaline and 2-(bromomethyl)-3-(2-chlorophenyl)-5- fluoroquinoxaline as a red syrup; LC-MS (ESI) two peaks of m/z 351.0 [M+H (79Br)]+and 352.9 [M+H (81Br)J+.
References:
WO2008/118468,2008,A1 Location in patent:Page/Page column 138-139
6305-95-9
101 suppliers
$13.00/1g

1064137-04-7
4 suppliers
inquiry
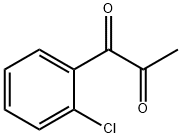
64497-33-2
6 suppliers
inquiry

1064137-04-7
4 suppliers
inquiry