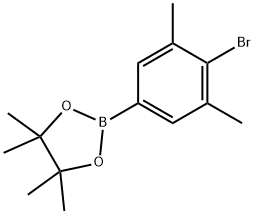
2-(4-bromo-3,5-dimethylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane synthesis
- Product Name:2-(4-bromo-3,5-dimethylphenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane
- CAS Number:1073338-97-2
- Molecular formula:C14H20BBrO2
- Molecular Weight:311.02
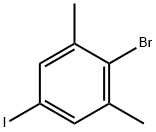
689260-53-5
107 suppliers
$9.00/250mg

73183-34-3
586 suppliers
$6.00/5g

1073338-97-2
18 suppliers
inquiry
Yield:-
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in dimethyl sulfoxide at 20 - 90; for 3 h;Inert atmosphere;
Steps:
1 Step 1:
2-bromo-1,3-dimethyl-5-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-benzene
Step 1:
2-bromo-1,3-dimethyl-5-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-benzene
A flask charged with a stir bar, 2-bromo-5-iodo-1,3-dimethyl-benzene (1.0 g), bis-(pinacolato)-diboron (1.0 g), potassium acetate (1.1 g) and dimethyl sulfoxide (10 mL) is purged with argon for 5 min. [1,1'-Bis(diphenylphosphino)-ferrocene]-dichloropalladium(II) (0.26 g) is added at room temperature, and the mixture is stirred at 90° C. for 3 h.
After cooling to room temperature, water is added and the resulting mixture is extracted with ethyl acetate.
The combined extracts are dried (MgSO4) and concentrated.
The residue is chromatographed on reversed phase (HPLC; acetonitrile/water) to give the title compound. LC (method 9): tR=1.30 min; Mass spectrum (ESI+): m/z=311/313 (Br) [M+H]+.
References:
US2013/252937,2013,A1 Location in patent:Paragraph 0863
576-22-7
328 suppliers
$10.00/1g

73183-34-3
586 suppliers
$6.00/5g

1073338-97-2
18 suppliers
inquiry