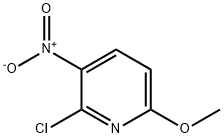
N-(2,2-Dimethyl-1,3-Dioxan-5-Yl)-6-Methoxy-3-Nitropyridin-2-Amine synthesis
- Product Name:N-(2,2-Dimethyl-1,3-Dioxan-5-Yl)-6-Methoxy-3-Nitropyridin-2-Amine
- CAS Number:1075237-91-0
- Molecular formula:C12H17N3O5
- Molecular Weight:283.28
Yield:1075237-91-0 92%
Reaction Conditions:
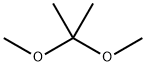
Stage #1: 2-{[6-(methyloxy)-3-nitro-2-pyridinyl]amino}-1,3-propanediol;2,2-dimethoxy-propanewith toluene-4-sulfonic acid at 20;
Stage #2: with water;sodium hydrogencarbonate for 0.333333 h;
Steps:
16A.b
(b) λ/-(2,2-Dimethyl-l,3-dioxan-5-yl)-6-(methyloxy)-3-nitro-2-pyridinamine; 2- { [6-(M ethyloxy)-3 -nitro-2-pyridinyl] amino} -1,3 -propanediol (53.93g,228.7mmol) was stirred in 2,2-dimethoxypropane (900ml) under argon and/?- toluenesulphonic acid monohydrate (1.0Og) was added. The mixture was stirred at room temperature overnight. This was diluted with dichloromethane (IL) and the resulting solution was treated with saturated aqueous sodium hydrogen carbonate (20ml) and solid sodium hydrogen carbonate (2Og) with vigorous stirring (effervescence). The mixture was vigorously stirred for 20 minutes, then the remaining water was absorbed by addition of anhydrous sodium sulphate. The mixture was filtered with suction and the solids were washed with DCM (500ml). The combined filtrate plus washings were evaporated under reduced pressure to give a yellow solid which was stirred with petroleum ether (40-60°) over the weekend. The solid was isolated by filtration with suction , washed with petroleum ether (40-60°) and air-dried to give the title compound as a bright yellow solid57.83g, 92%).MS (ES+) m/z 284 (MH+).
References:
WO2008/128942,2008,A1 Location in patent:Page/Page column 64
38533-61-8
310 suppliers
$8.00/5g

1075237-91-0
13 suppliers
inquiry
![1,3-Propanediol, 2-[(6-methoxy-3-nitro-2-pyridinyl)amino]-](/CAS/20210305/GIF/757186-96-2.gif)