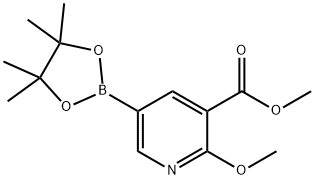
Methyl2-methoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)nicotinate synthesis
- Product Name:Methyl2-methoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)nicotinate
- CAS Number:1083168-93-7
- Molecular formula:C14H20BNO5
- Molecular Weight:293.12
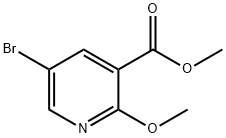
122433-41-4

73183-34-3

1083168-93-7
General procedure for the synthesis of methyl 2-methoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)nicotinate from 5-bromo-2-methoxynicotinic acid methyl ester and pinacol ester of bisboronic acid: to a dry flask were added 5-bromo-2-methoxynicotinic acid methyl ester (0.5 g, 2.0 mmol), pinacol ester of bisboronic acid (0.61 g, 2.4 mmol) and Pd(dppf)Cl2 (82 mg, 0.10 mmol). Subsequently, potassium acetate (0.6 g, 6.0 mmol) was added directly to the flask. The flask was evacuated and backfilled three times with nitrogen. Anhydrous N,N-dimethylformamide (10.0 mL) was added and the reaction mixture was heated in an oil bath at 80°C overnight. After completion of the reaction, the mixture was evaporated to dryness and the residue was dissolved in ethyl acetate (10 mL) and washed with water (10 mL). The organic phase was dried with anhydrous sodium sulfate and then evaporated to dryness. The crude product was purified by silica gel column chromatography using a hexane solution of 0-70% ethyl acetate as eluent to give the target product (0.36 g in 72% yield).The calculated value for ESI-MS m/z was 249.11, and the measured value was 250.3 ([M+H]+).The HPLC retention time was 1.84 min.

122433-41-4
117 suppliers
$25.00/1g

73183-34-3
587 suppliers
$6.00/5g

1083168-93-7
50 suppliers
$55.00/25mg
Yield:1083168-93-7 70.9%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;potassium acetate in 1,4-dioxane at 65;
Steps:
2.2C
2C: Methyl 2-methoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)nicotinate : A degassed solution of methyl 5-bromo-2-methoxynicotinate (1.25 g, 5.08 mmol), 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi(1,3,2-dioxaboi lane) (1.806 g, 7.11 mmol), potassium acetate (0.773 g, 7.87 mmol) and PdCl2(dppf)-CH2Cl2 adduct (0.332 g, 0.406 mmol) in dioxane (15 mL) was heated to 65 °C with vigorous mixing ON. The reaction mixture was concentrated onto Celite and purified by flash chomatography utilizing a 40 g ISCO column, eluting with 0-90% EtOAc in hexanes. Concentration of the pure fractions afforded methyl 2-methoxy-5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)nicotinate (1.111 g, 3.60 mmol, 70.9 % yield). MSESI m/z 294.1 (M+H)
References:
WO2019/89442,2019,A1 Location in patent:Page/Page column 56

67367-26-4
92 suppliers
$19.00/5g

73183-34-3
587 suppliers
$6.00/5g

1083168-93-7
50 suppliers
$55.00/25mg

67367-26-4
92 suppliers
$19.00/5g

73183-34-3
587 suppliers
$6.00/5g

1083168-93-7
50 suppliers
$55.00/25mg
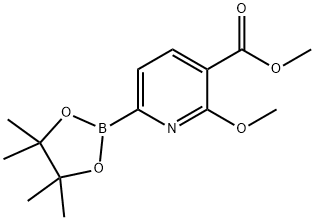
1246765-27-4
24 suppliers
$410.88/1g