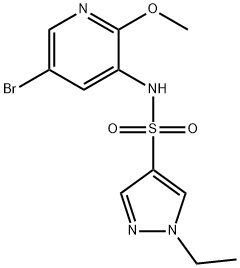
N-(5-broMo-2-Methoxypyridin-3-yl)-1-ethyl-1H-pyrazole-4-sulfonaMide synthesis
- Product Name:N-(5-broMo-2-Methoxypyridin-3-yl)-1-ethyl-1H-pyrazole-4-sulfonaMide
- CAS Number:1083326-09-3
- Molecular formula:C11H13BrN4O3S
- Molecular Weight:361.21
884495-39-0
172 suppliers
$5.00/1g

957514-21-5
43 suppliers
$35.00/250mg

1083326-09-3
3 suppliers
inquiry
Yield:1083326-09-3 77%
Reaction Conditions:
with pyridine at 20; for 1.5 h;
Steps:
5
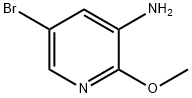
Intermediate 5; Preparation of N-[5-bromo-2-(methyloxy)-3-pyridinyl]-1-ethyl-1H-pyrazole-4-sulfonamide; In an oven dried round bottom flask under a nitrogen atmosphere, a solution of 5-bromo-2-(methyloxy)-3-pyridinamine (268 mg, 1.320 mmol) in anhydrous pyridine (4 mL) was treated with 1-ethyl-1H-pyrazole-4-sulfonyl chloride (308 mg, 1.584 mmol) and the resultant reaction mixture stirred at room temperature for 90 min. The reaction mixture was concentrated in vacuo, diluted with ethyl acetate (100 mL) and water (30 mL), neutralized with saturated ammonium chloride (aq) and the product extracted into the organic layer. The aqueous layer was back-extracted with 40 mL ethyl acetate. The organic layers were combined, washed with brine (50 mL), dried over magnesium sulfate and concentrated in vacuo to give a yellow-brown solid. Purification by silica gel chromatography (0-50% ethyl acetate in hexanes) provided the title compound (368 mg, 77%) as a beige solid. MS (ES)+ m/e 361.0, 363.0 [M+H]+.
References:
US2008/293706,2008,A1 Location in patent:Page/Page column 86