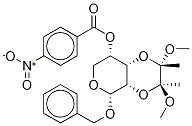
Benzyl 2,3-O-[(1S,2S)-1,2-Dimethoxy-1,2-dimethyl-1,2-ethanediyl]-4-nitrobenzoyl-α-L-xylopyranoside synthesis
- Product Name:Benzyl 2,3-O-[(1S,2S)-1,2-Dimethoxy-1,2-dimethyl-1,2-ethanediyl]-4-nitrobenzoyl-α-L-xylopyranoside
- CAS Number:1084896-42-3
- Molecular formula:C25H29NO10
- Molecular Weight:503.5
![Benzyl 2,3-O-[(1S,2S)-1,2-Dimethoxy-1,2-dimethyl-1,2-ethanediyl]-β-D-arabinopyranoside](/CAS/GIF/887370-09-4.gif)
887370-09-4
14 suppliers
$110.50/50mg
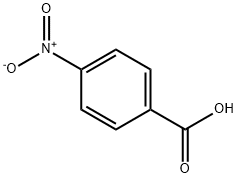
62-23-7
734 suppliers
$14.00/25g
![Benzyl 2,3-O-[(1S,2S)-1,2-Dimethoxy-1,2-dimethyl-1,2-ethanediyl]-4-nitrobenzoyl-α-L-xylopyranoside](/CAS/GIF/1084896-42-3.gif)
1084896-42-3
15 suppliers
inquiry
Yield:1084896-42-3 86%
Reaction Conditions:
with di-isopropyl azodicarboxylate;triphenylphosphine in tetrahydrofuran at 40 - 62; for 17 h;
Steps:
1
(2S,3S,4aS,5R,8R,8aR)-5-(benzyloxy)-2,3-dimethoxy-2,3-dimethylhexahydro-2H-pyrano[4,3-b][l,4]dioxin-8-ol (2, 8 g, 0.023 mol), triphenylphosphine (11.84 g, 0.045 mol), and 4-nitrobenzoic acid (7.54 g, 0.045 mol) were mixed in THF (80 mL) under nitrogen and heated to 40 0C. Diisopropylazodicarboxylate (9.13 g, 0.045 mol) was added dropwise, then the mixture was heated to 62 0C for 17 h. The reaction was cooled to room temperature, solvent was evaporated, and the product (8) was crystallized from methanol (86%). m.p. 170 - 171 0C. 1H NMR (300 MHz, CDCl3): δ 8.21 (d, J = 9 Hz, 2H), 8.10 (d, J = 9 Hz, 2H), 7.37 - 7.19 (m, 5H), 5.14 (m, IH), 4.85 (d, J = 3.6 Hz, IH), 4.71 (d, J = 12.6 Hz, IH), 4.61 (d, J = 12.6 Hz, IH), 4.31 (t, J = 9.9 Hz, IH), 3.89 - 3.77 (m, 2H), 3.58 (t, J = 10.6 Hz, IH), 3.39 (s, 3H), 3.21 (s, 3H), 1.28 (s, 3H), 1.19 (s, 3H).
References:
WO2008/144773,2008,A1 Location in patent:Page/Page column 30