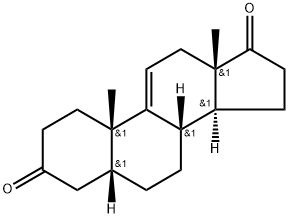
1093397-63-7 synthesis
- Product Name:1093397-63-7
- CAS Number:1093397-63-7
- Molecular formula:C19H26O2
- Molecular Weight:286.42
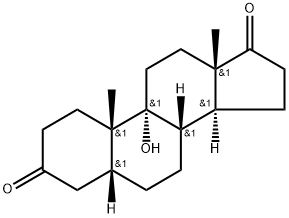
1093397-61-5
1 suppliers
inquiry

1093397-63-7
13 suppliers
inquiry
Yield:1093397-63-7 95%
Reaction Conditions:
with sulfuric acid in dichloromethane at 10 - 35; for 2.15 h;Product distribution / selectivity;
Steps:
8; 11
Experimental procedure:01. Charge dichloromethane (1275 mL) and the Stage-I product into a clean and dry flask.02. Cool the mixture to 10 °C and then add sulfuric acid (13.5 mL) slowly over 15 min at 10-15°C.03. Reaction solution temp raise to 25-35°C and stir for 2 h at 25-35°C.04. Check for completion by TLC (30% EtOAc in DCM; NMT 1 % of Stage-I product).05. Wash the reaction mixture with water (300 mL).06. Back extract the aqueous layer with DCM (2 X212 mL), and then combine the organic layers.07. Wash the organic layer with saturated sodium bicarbonate solution (425 mL)08. Wash the organic layer with water (550 mL) followed by brine solution (425 mL).07. Distill out the solvent completely under vacuum at less than 45 °C.08. Add hexanes (340 mL) and distill out the solvent completely under vacuum at less than 50 °C.09. Add water (616 mL), stir for 15 min at RT, then filter and wash the cake with water (255 mL).10. Dry the white solid in a hot air drier at 55-60 °C until the moisture content is NMT 0.5%.Wet weight: -190 g. Dry weight: 76 gYield: 95%Moisture < 0.5%Melting range: 148.5 - 150.1°CSOR: +144.4 (c = 1% in CHCl3).HPLC/ RI Purity: 96.0%. TLC: p-Anisaldehyde charring, Rf for compound 27 = 0.76 and Rf for compound 25 = 0.44. Eluent was 30% EtOAc in DCM.1H NMR (500MHz , CDC13 ): δ = 5.61 (s , 1H ), 2.57-2.47(m, 2H), 2.42-2.24 (m, 4H), 2.20-2.05 (m, 3H), 1.99-1.86 (m, 2H), 1.85-1.84 (d, J = 6 Hz 1H), 1.63-1.57 (m, 5H), 1.40-1.37(d, J = 13.5 Hz, 1H) 1.28-1.25 (dd, J = 4.0, 13.5 Hz, 1H), 1.17 (s, 3H) 0.85 (s, 3H).13C NMR (125 MHz, CDC13): δ = 221.3, 212.8, 140.1, 1 18.5, 48.5, 45.9, 44.3, 43.5, 39.0, 38.0, 37.3, 36.1 , 35.8, 33.3, 28.8, 26.0, 25.5, 22.5, 13.9.Mass (m/z) = 287 [M+ + 1 ], 304 [ M+ + 18 ].IR (KBr) = 3450, 2913, 1737, 1707,1413, 1403,1207 cm-1.m.p. = 143.4-145.9 °C (from DCM).[α]D = +142 (c = 1% in CHCl3).HPLC/ RI Purity: 96.7%.
References:
WO2011/75701,2011,A2 Location in patent:Page/Page column 77-78, 103-104, 125-126
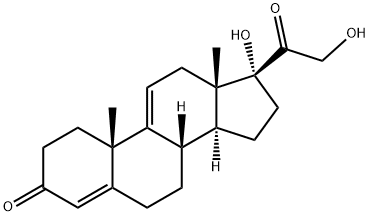
10184-70-0
26 suppliers
$295.00/10mg

1093397-63-7
13 suppliers
inquiry