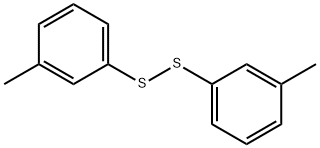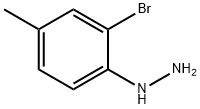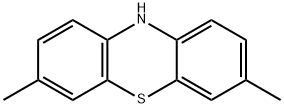
10H-Phenothiazine, 3,7-dimethyl- synthesis
- Product Name:10H-Phenothiazine, 3,7-dimethyl-
- CAS Number:20751-71-7
- Molecular formula:C14H13NS
- Molecular Weight:227.32
Yield:20751-71-7 95.4%
Reaction Conditions:
with 1,4-diaza-bicyclo[2.2.2]octane;1-(2-hydroxyethyl)-2,3-dimethylimidazolium hexafluorophosphate;1,1'-bis(diisopropylphosphino)ferrocene;tetracarbonyl nickel;4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in N,N-dimethyl-formamide;chlorobenzene at 80; for 8 h;Reagent/catalyst;Solvent;
Steps:
3 Example 3
To a suitable amount of an organic solvent (a mixture of N, N-dimethylformamide (DMF) and chlorobenzene in a volume ratio of 2: 1) was added at room temperature 100 mmol of the compound of the above formula (I), 170 mmol of the compound of the above formula , 15 mmol catalyst (3.3 mmol 1, 1'-bis (diisopropylphosphino) ferrocene and 11.7 mmol of tetracarbonyl nickel), 11 mmol of ligand L1, 130 mmol of a baseDABCO and 15 mmol of the additive 1,2-dimethyl-3-hydroxyethylimidazolium hexafluorophosphate, and then the temperature was raised to 80 ° C and at this temperature The reaction was stirred for 8 hours;After the reaction is completed, deionized water is added to the reaction mixture to sufficiently shake, and then extracted with ethyl acetate for 2-3 times, The organic phases are combined and washed with saturated brine for 2-3 times, the organic phases are separated and combined, dried over anhydrous magnesium sulphate, concentrated under reduced pressure, The residue was separated on a silica gel column and eluted with a 1: 2 by volume mixture of chloroform and petroleum ether to give the compound of the above formula(III) was obtained in a yield of 95.4%.
References:
CN106279063,2017,A Location in patent:Paragraph 0049; 0050; 0051-0053; 0060; 0061; 0065-0066
620-93-9
267 suppliers
$29.00/5g

20751-71-7
1 suppliers
inquiry