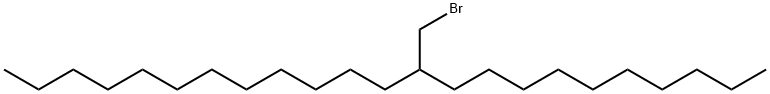
11-(bromomethyl)tricosane synthesis
- Product Name:11-(bromomethyl)tricosane
- CAS Number:732276-63-0
- Molecular formula:C24H49Br
- Molecular Weight:417.55
58670-89-6
102 suppliers
$36.00/25ML

732276-63-0
62 suppliers
$148.00/5ML
Yield:732276-63-0 98%
Reaction Conditions:
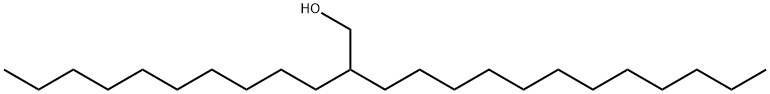
Stage #1: 2-decyltetradecyl alcoholwith triphenylphosphine in dichloromethane at 0; for 0.0833333 h;Inert atmosphere;
Stage #2: with NBS in dichloromethane; for 16 h;
Steps:
1 Example 1 Synthesis of 1 -bromo-2-decyltetradecane (1)
Under argon, triphenylphosphine (27.8 g, 70.5 mmol) was suspended in a flask with DCM (47 ml). The mixtures was cooled to 0°C before 2-decyltetradecan-1-ol (29.8 ml, 105.8 mmol) was introduced. After 5 minutes stirring, N-bromosuccinimide (18.8 g, 105.8 mmol) was added poration wise to the flask. The reaction mixture immediately turned yellow and continued to darken to orange. The reaction was stirred for 16 hours after which the solvent was removed by vacuum evaporation. The brown residue was diluted with petroleum ether and the solution flushed through a silica plug. The filtrate was evaporated to give a clear oil. Yield: 28.7 g, 98 %. 1H NMR(400 MHz, Chloroform-d) 6 3.44 (d, J = 4.7 Hz, 2H), 1.66 - 1.47 (m, 2H), 1.44 - 1.15 (m, 40H),0.88 (t, J= 6.6 Hz, 7H).13C NMR (101 MHz, ODd3) 639.72,39.70,32.75,32.10,29.97,29.82,29.77, 29.53, 26.74, 22.86, 14.26.
References:
WO2017/148864,2017,A1 Location in patent:Page/Page column 36; 37