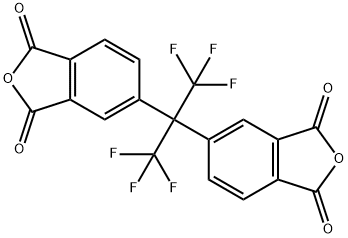
4,4'-(Hexafluoroisopropylidene)diphthalic anhydride synthesis
- Product Name:4,4'-(Hexafluoroisopropylidene)diphthalic anhydride
- CAS Number:1107-00-2
- Molecular formula:C19H6F6O6
- Molecular Weight:444.24

108-24-7
65294-20-4

1107-00-2
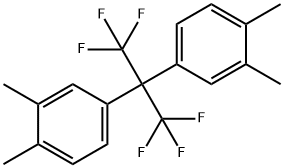
The general procedure for the synthesis of hexafluorodianhydride (6FDA) from ethanoic anhydride and 2,2-bis(3,4-dimethylphenyl)hexafluoropropane was as follows: in a 2000 mL three-necked flask, 14.40 g of 4,4'-(hexafluoroisopropylidene)bis(o-xylene) and solvent mixture of pyridine (200 mL) and water (100 mL) were added. The mixture was heated to 100 °C and then 38.0 g of potassium permanganate was added slowly and the reaction was maintained for 3 hours. Upon completion of the reaction, a small amount of ethanol was added dropwise to quench the unreacted potassium permanganate. The reaction mixture was filtered and the pyridine was subsequently recovered from the filtrate by distillation. The pH of the remaining solution was adjusted to 1 and water was removed to dryness by evaporation. 320 mL of acetone was added to dissolve the organic material, and after filtration to remove insoluble material, the filtrate was evaporated to dryness to give hexafluorobutyric acid in 80% yield. A mixture of 4.80 g of hexafluorobutyric acid with 8 mL of acetic anhydride and 8 mL of xylene was added to a 150 mL flask and reacted at 140 °C for 40 min. After completion of the reaction, the crude product was cooled and filtered to obtain hexafluorodianhydride (6FDA) crude product. After purification by sublimation and drying, hexafluorodianhydride (6FDA) with purity greater than 99.5% was obtained in 79% yield.
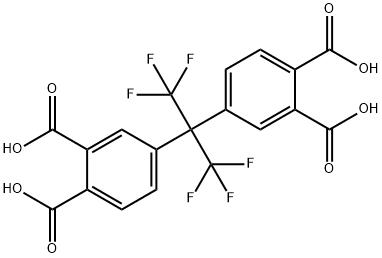
3016-76-0
117 suppliers
$6.00/1g

1107-00-2
418 suppliers
$6.00/1g
Yield:1107-00-2 96%
Reaction Conditions:
with acetic anhydride at 120; for 4 h;Temperature;
Steps:
1.2; 15-16; 1-2
Add hexafluorotetraacid to 40ml of anhydrous acetic anhydride, stir and reflux at 120°C for 4h, the molar ratio of hexafluorotetraacid to anhydrous acetic anhydride is 1:3.5. The reaction solution was cooled by an ice-water bath to precipitate white crystals, and after drying, the crude hexafluorodianhydride was obtained with a purity of 98%.The crude hexafluorodianhydride was added to 20ml of anhydrous trifluoroacetic anhydride and mixed, and then heated to reflux at 35°C for 1.5h. The molar ratio of crude hexafluorodianhydride to anhydrous trifluoroacetic anhydride was 1:1.5. The reaction solution was cooled by an ice-water bath to precipitate white crystals, and after drying, the finished product of hexafluorodianhydride was obtained with a purity of 99.8%. The yield of S2 step was 96%.
References:
CN113480504,2021,A Location in patent:Paragraph 0026; 0030-0031; 0045-0046; 0047-0048; 0049; 0051

65294-20-4
202 suppliers
$5.00/1g

1107-00-2
418 suppliers
$6.00/1g

108-24-7
0 suppliers
$14.00/250ML

65294-20-4
202 suppliers
$5.00/1g

1107-00-2
418 suppliers
$6.00/1g
![Phenol, 4,4'-[2,2,2-trifluoro-1-(trifluoromethyl)ethylidene]bis[2-nitro-](/CAS/20180531/GIF/73340-33-7.gif)
73340-33-7
21 suppliers
inquiry

1107-00-2
418 suppliers
$6.00/1g
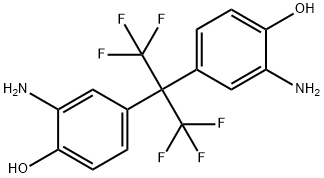
83558-87-6
366 suppliers
$8.00/5g

1107-00-2
418 suppliers
$6.00/1g