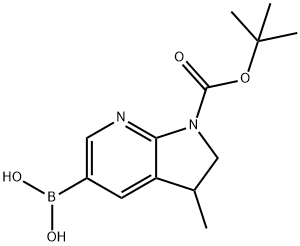
1H-Pyrrolo[2,3-b]pyridine-1-carboxylic acid, 5-borono-2,3-dihydro-3-Methyl-, 1-(1,1-diMethylethyl) este synthesis
- Product Name:1H-Pyrrolo[2,3-b]pyridine-1-carboxylic acid, 5-borono-2,3-dihydro-3-Methyl-, 1-(1,1-diMethylethyl) este
- CAS Number:1111637-67-2
- Molecular formula:C13H19BN2O4
- Molecular Weight:278.11
![1H-Pyrrolo[2,3-b]pyridine-1-carboxylic acid, 5-broMo-2,3-dihydro-3-Methyl-, 1,1-diMethylethyl ester](/CAS/GIF/1111637-66-1.gif)
1111637-66-1
8 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridine-1-carboxylic acid, 5-borono-2,3-dihydro-3-Methyl-, 1-(1,1-diMethylethyl) este](/CAS/20180808/GIF/1111637-67-2.gif)
1111637-67-2
1 suppliers
inquiry
Yield:1111637-67-2 51%
Reaction Conditions:
Stage #1: C13H17BrN2O2with n-butyllithium;Triisopropyl borate in tetrahydrofuran;hexanes at -78; for 2 h;
Stage #2: with hydrogenchloride;water at 0; pH=7;
Steps:
7
Step 7:To a stirring solution of compound 63 (460 mg, 1.47 mmol) and triisopropylborate (691 mg, 3.67 mmol) in 25 mL dry THF at -78 ° C was added Butyl lithium solution (2.5 M in hexanes, 1.47 mL, 3.67 mmol) dropwise under nitrogen. The reaction was stirred at -78 ° C and monitored with LCMS. 2hr later, LCMS indicated reaction complete. Reaction was quenched with 25 mL water and concentrated under reduced pressure to a total volume of about 15 mL. The residual was washed with ether (2X10 mL) and the
References:
WO2009/16460,2009,A2 Location in patent:Page/Page column 50-51