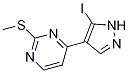
4-(5-iodo-1H-pyrazol-4-yl)-2-(methylthio)pyrimidine synthesis
- Product Name:4-(5-iodo-1H-pyrazol-4-yl)-2-(methylthio)pyrimidine
- CAS Number:1111637-86-5
- Molecular formula:C8H7IN4S
- Molecular Weight:318.1375
956722-10-4
20 suppliers
$45.00/2.5mg

1111637-86-5
11 suppliers
inquiry
Yield:1111637-86-5 54.6%
Reaction Conditions:
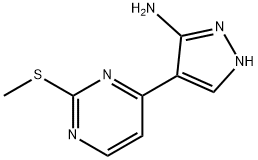
Stage #1: 4-(2-(methylthio)pyrimidin-4-yl)-1H-pyrazol-5-aminewith sulfuric acid;iodine;acetic acid;potassium iodide;sodium nitrite in water at -3 - 50; for 2 h;
Stage #2: with ammonia;water
Steps:
A-1
Preparation of 4-(5-iodo-1 H-pyrazol-4-yl)-2-(methylthio)pyrimidine (A)A solution of NaNO2 (20.0 g, 0.29 mol) in water (150 mL) was poured into a solution of 4-(2- (methylthio)pyrimidin-4-yl)-1 H-pyrazol-5-amine 6 (50.0 g, 0.24 mol) in a mixture of glacial acetic acid (400 mL) and water (100 mL) at -3 0C. The temperature increased to -1 0C. Concentrated H2SO4 (10 mL) was added to the obtained solution, and a solution of potassium iodide (120.0 g, 1.2 mol.) and I2 (123.0 g, 10.48 mol) in water (200 mL) was added dropwise. The obtained solution was heated to 50 0C for 2 h, and the mixture was neutralized with aqueous ammonia. Excess iodine was treated with Na2S2O3. The precipitate was filtered, and the filtrate was extracted with ethyl acetate. The organic layer was evaporated, and the residue was purified by chromatography (THF: EtOAc = 4:1 ) to give 4-(5-iodo-1 H- pyrazol-4-yl)-2-(methylthio)pyrimidine (A) (42.2 g, 54.6%) as a yellow solid. 1H NMR (400 MHz, DMSO): δ 8.55 (d, 1 H), 8.35 (s, 1H), 7.55 (d, 1 H), 2.55 (s, 3H).
References:
WO2009/16460,2009,A2 Location in patent:Page/Page column 40; 59