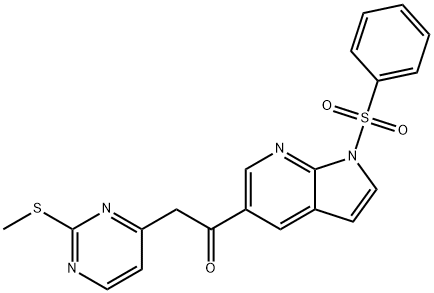
2-[2-(Methylthio)pyrimidin-4-yl]-1-[1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridin-5-yl]ethanone synthesis
- Product Name:2-[2-(Methylthio)pyrimidin-4-yl]-1-[1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridin-5-yl]ethanone
- CAS Number:1111638-52-8
- Molecular formula:C20H16N4O3S2
- Molecular Weight:424.5
14001-63-9
136 suppliers
$17.00/1g
![1-Benzenesulfonyl-1H-pyrrolo[2,3-b]pyridine-5-carboxylic acid methyl ester](/CAS/GIF/1083181-12-7.gif)
1083181-12-7
19 suppliers
inquiry
![2-[2-(Methylthio)pyrimidin-4-yl]-1-[1-(phenylsulfonyl)-1H-pyrrolo[2,3-b]pyridin-5-yl]ethanone](/CAS2/GIF/1111638-52-8.gif)
1111638-52-8
10 suppliers
$505.42/5MG
Yield:1111638-52-8 58.5%
Reaction Conditions:
Stage #1: 2-methylthio-4-methylpyrimidinewith n-butyllithium;diisopropylamine in tetrahydrofuran at -78; for 1 h;
Stage #2: 1-benzenesulfonyl-1H-pyrrolo[2,3-b]pyridin-5-carboxylic acid methyl ester in tetrahydrofuran at -110; for 0.166667 h;Product distribution / selectivity;
Steps:
G-1
Preparation of 2-(2-(methylthio)pyrimidin-4-yl)-1 -(1 -(phenylsulfonyl)-i H-pyrrolo[2,3-b]pyridin-5- yl)ethanone (G-1-3)n-BuLi (2.5 M, 93 mL, 0.233 mol) was added drop-wise to a solution of i-Pr2NH (32.5 mL, 0.233 mol) in dry THF (420 mL) at -780C and the resulting solution was stirred at -780C for 30 min. Then, a solution of 4- methyl-2-(methylthio)pyrimidine (22.33 g, 0.16 mol) in dry THF (110 mL) was added drop-wise and the resulting mixture was stirred at -780C for another 30 min. A solution of compound G-1-2 (50.0 g, 0.145 mol) in dry THF (250 mL) was then added drop-wise at -11O0C. After the addition, the resulting mixture was stirred at -11O0C for 10 min. TLC (hexane: EtOAc 1 :1 ) indicated the reaction was complete. EtOAc (300 mL) and H2O (300 mL) were added to the reaction mixture to quench the reaction. The organic layer was separated and the aq. layer was extracted with EtOAc (300 mLx3). The combined organic layers were washed with saturated aqueous NaCI (500 mL), dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography (CH2CI2) to give compound 7 (36.0 g, 58.5%) as a yellow solid.
References:
WO2009/16460,2009,A2 Location in patent:Page/Page column 112