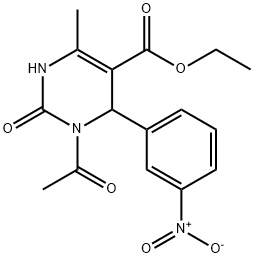
ethyl 1-acetyl-4-methyl-6-(3-nitrophenyl)-2-oxo-1,2,3,6-tetrahydropyrimidine-5-carboxylate synthesis
- Product Name:ethyl 1-acetyl-4-methyl-6-(3-nitrophenyl)-2-oxo-1,2,3,6-tetrahydropyrimidine-5-carboxylate
- CAS Number:111535-67-2
- Molecular formula:C16H17N3O6
- Molecular Weight:347.32

111535-69-4
0 suppliers
inquiry

111535-67-2
7 suppliers
inquiry
Yield:-
Reaction Conditions:
in acetic anhydride;
Steps:
1.b (b)
(b) Ethyl 3-acetyl-6-methyl-4-(3-nitrophenyl)-3,4-dihydropyrimidine-2(1H)one-5-carboxylate Compound 1a (1.00 gr.) in acetic anhydride (25 mL) was heated to reflux for 45 minutes. The reaction mixture was cooled and concentrated in vacuo to give a tan solid. The tan solid was purified by flash chromatography on silica gel eluted with methanol:methylene chloride (2:98) and two recrystallizations from toluene to yield the desired product 1b (m.p. 190°-190.5° C.). 1H NMR(360 MHz,CDCl3),δ=6.62 (S, 1H, ArCH).
References:
US4675321,1987,A