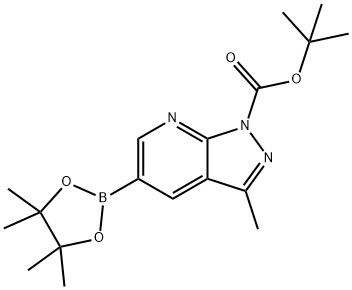
tert-butyl 3-Methyl-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazolo[3,4-b]pyridine-1-carboxylate synthesis
- Product Name:tert-butyl 3-Methyl-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazolo[3,4-b]pyridine-1-carboxylate
- CAS Number:1131121-50-0
- Molecular formula:C18H26BN3O4
- Molecular Weight:359.23
Yield:1131121-50-0 50%
Reaction Conditions:
with potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in 1,4-dioxane at 80; for 4 h;
Steps:
1.c
A mixture of 1 ,1-dimethylethyl 3-methyl-5-{[(trifluoromethyl)sulfonyl]oxy}-1/-/- pyrazolo[3,4-6]pyridine-1-carboxylate (ref. PCT Int. Appl. (2005), WO 2005085227) (1.3g, 3.41 mmol), 4,4,4',4',5,5,5',5'-octamethyl-2,2'-bi-1 ,3,2-dioxaborolane (1.13g, 4.43 mmol), potassium acetate (0.5g, 5.1 1 mmol), Pd(dppf)CI2 CH2CI2 (140mg, 0.17 mmol) and dioxane (20 mL) was charged into a 50 ml sealed flask. The reaction was heated at 80 0C for 4 hours. The reaction mixture was filtered and the solvent was removed under vacuum. The residue was purified on Biotage (30% EtOAc/hexane) to give 0.62g of 1c as a white solid (50%).
References:
WO2009/32652,2009,A1 Location in patent:Page/Page column 8; 32
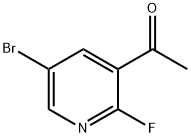
1111637-74-1
80 suppliers
$15.00/250mg
![tert-butyl 3-Methyl-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazolo[3,4-b]pyridine-1-carboxylate](/CAS/20150408/GIF/1131121-50-0.gif)
1131121-50-0
10 suppliers
inquiry
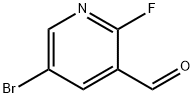
875781-15-0
182 suppliers
$6.00/1g
![tert-butyl 3-Methyl-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazolo[3,4-b]pyridine-1-carboxylate](/CAS/20150408/GIF/1131121-50-0.gif)
1131121-50-0
10 suppliers
inquiry

1111637-73-0
26 suppliers
inquiry
![tert-butyl 3-Methyl-5-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazolo[3,4-b]pyridine-1-carboxylate](/CAS/20150408/GIF/1131121-50-0.gif)
1131121-50-0
10 suppliers
inquiry
![1H-Pyrazolo[3,4-b]pyridine-1-carboxylic acid, 3-methyl-5-[[(trifluoromethyl)sulfonyl]oxy]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/864775-99-5.gif)
