
6-Bromo-2,4-dichloro-7-(phenylsulfonyl)-7H-Pyrrolo[2,3-d]pyrimidine synthesis
- Product Name:6-Bromo-2,4-dichloro-7-(phenylsulfonyl)-7H-Pyrrolo[2,3-d]pyrimidine
- CAS Number:1131992-26-1
- Molecular formula:C12H6BrCl2N3O2S
- Molecular Weight:407.07
![2,4-Dichloro-7-(phenylsulfonyl)-7H-Pyrrolo[2,3-d]pyrimidine](/CAS/GIF/1131992-22-7.gif)
1131992-22-7
35 suppliers
$60.00/100mg
![6-Bromo-2,4-dichloro-7-(phenylsulfonyl)-7H-Pyrrolo[2,3-d]pyrimidine](/CAS/GIF/1131992-26-1.gif)
1131992-26-1
21 suppliers
inquiry
Yield:1131992-26-1 71%
Reaction Conditions:
Stage #1: 7-(benzenesulfonyl)-2,4-dichloro-pyrrolo[2,3-d]pyrimidinewith lithium diisopropyl amide in tetrahydrofuran at -78 - 0; for 0.5 h;
Stage #2: with 1,2-dibromo-1,1,2,2-tetrachloroethane in tetrahydrofuran at -78; for 2 h;
Steps:
32; 4
A solution of LDA in THF, prepared at 0 0C by addition of n-BuLi (2.5 M in hexanes, 2.5 ml_, 6.378 mmol) to a solution Of Z-Pr2NH (980 μl_, 6.932 mmol) in 5 mL of anhydrous THF, cooled to -78 0C was treated with a solution of Example 31 (910 mg, 2.773 mmol) in 10 mL THF. After 30 min, the reaction mixture was treated with a solution of 1 ,2-dibromo-tetrachloroethane (2.709 g, 8.319 mmol) in 10 mL THF. After 2 h at -78 0C, the reaction mixture was quenched with sat NH4CI(aq) (20 mL). The aqueous layer was extracted with EtOAc (2x). Organics were combined, dried (MgSO4), filtered and the volatiles were removed in vacuo. The residue was purified by flash chromatography (50 g lsolute SiO2 cartridge, gradient hexanes 100% to hexanes/EtOAc, 4:1 ) to provide the desired product (805 mg, 71 %) as a colourless solid. 1H NMR (CDCI3) δ 8.26-8.23 (2H, m), 7.68 (1 H, dt, J = 1.2, 7.6 Hz), 7.60-7.56 (2H, m), 6.81 (1 H, s).
References:
WO2009/37467,2009,A1 Location in patent:Page/Page column 33; 15
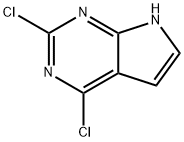
90213-66-4
495 suppliers
$8.00/250mg
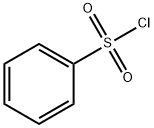
98-09-9
364 suppliers
$12.00/25g
![6-Bromo-2,4-dichloro-7-(phenylsulfonyl)-7H-Pyrrolo[2,3-d]pyrimidine](/CAS/GIF/1131992-26-1.gif)
1131992-26-1
21 suppliers
inquiry