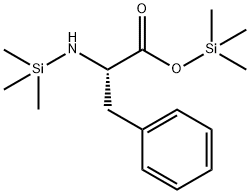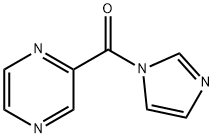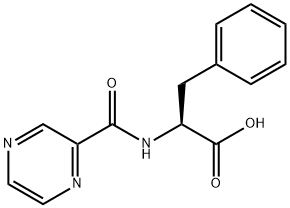
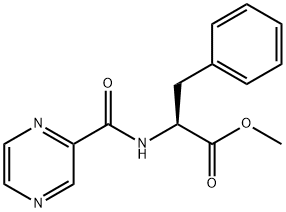
(S)-3-PHENYL-2-[(PYRAZIN-2-YLCARBONYL)AMINO] PROPANOIC ACID synthesis
- Product Name:(S)-3-PHENYL-2-[(PYRAZIN-2-YLCARBONYL)AMINO] PROPANOIC ACID
- CAS Number:114457-94-2
- Molecular formula:C14H13N3O3
- Molecular Weight:271.27
73058-37-4
![(S)-3-PHENYL-2-[(PYRAZIN-2-YLCARBONYL)AMINO] PROPANOIC ACID](/CAS/GIF/114457-94-2.gif)
114457-94-2
General procedure for the synthesis of (S)-3-phenyl-2-(pyrazine-2-carboxamido)propionic acid from (pyrazine-2-carbonyl)-L-phenylalanine methyl ester: N-pyrazinecarbonyl-L-phenylalanine methyl ester (1.0 g, 3.51 mmol) was dissolved in 10 mL of acetone, and the pH was adjusted to 12-13 by slowly adding 2N NaOH solution dropwise under cooling in an ice-water bath.Reaction. The progress of the reaction was monitored by thin-layer chromatography (TLC) during the process, and the reaction was completed after 2 hours. Subsequently, hydrochloric acid was slowly added dropwise in an ice-water bath and the pH was adjusted to 2~3, at which time a large amount of white solid precipitated. The precipitate was collected by filtration, washed with water and ether sequentially, and then dried in air. Finally, 0.89 g of white solid product was obtained with a yield of 93.6% and a melting point of 166-169°C. The product structure was analyzed by 1H-NMR. The structure of the product was confirmed by 1H-NMR (DMSO-d6, 300 MHz): δ 3.23 (-CH2, m, 2H), 4.74 (-CH, m, 1H), 7.16-7.25 (-Ph, m, 5H), 8.74 (-CONH, t, 1H), 8.86-8.89 (-Pyz, t, 2H), 9.14 (-Pyz. d, 1H), 13.06 (-COOH, s, 1H).

73058-37-4
64 suppliers
$230.00/100mg
![(S)-3-PHENYL-2-[(PYRAZIN-2-YLCARBONYL)AMINO] PROPANOIC ACID](/CAS/GIF/114457-94-2.gif)
114457-94-2
336 suppliers
$5.00/250mg
Yield:114457-94-2 93.6%
Reaction Conditions:
with sodium hydroxide in acetone; pH=12 - Ca. 13Cooling with ice;
Steps:
3 Preparation example 3 N-pyrazineformyl-L-phenylalanine
The product N-pyrazineformyl-L-phenylalanine methyl ester (1.0g, 3.51mmol) in Preparation example 2 was dissolved with 10ml of acetone, 2N NaOH was added dropwise slowly until a pH value of 12∼13 was obtained, and the solution was kept reacting under the condition of ice water bath, the reaction was monitored by TLC and completed after 2h.
Hydrochloric acid was add dropwise under the condition of ice water bath until a pH value of 2∼3 was obtained, a large amount of white solid was produced, the generated precipitate was filtered, washed with water and diethyl ether followed by airing to dry and gave 0.89g of white product with a yield of 93.6%, m.p.: 166-169°C. 1H-NMR (DMSO-d6, 300MHz): δ 3.23 (-CH2, m, 2H), 4.74 (-CH, m, 1H), 7.16∼7.25 (-Ph, m, 5H), 8.74 (-CONH, t, 1H), 8.86∼8.89 (-Pyz, t, 2H), 9.14 (-Pyz, d, 1H), 13.06 (-COOH, s, 1H).
References:
Peking University;LI, Runtao;CUI, Jingrong;ZHU, Yongqiang;YAO, Shuyang;GE, Zemei;CHENG, Tieming EP2444411, 2016, B1 Location in patent:Paragraph 0038
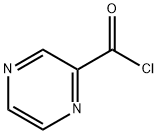
19847-10-0
75 suppliers
$95.00/1g
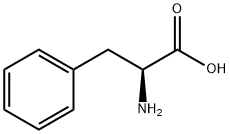
63-91-2
933 suppliers
$10.00/100G
![(S)-3-PHENYL-2-[(PYRAZIN-2-YLCARBONYL)AMINO] PROPANOIC ACID](/CAS/GIF/114457-94-2.gif)
114457-94-2
336 suppliers
$5.00/250mg
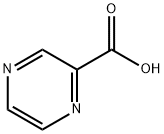
98-97-5
443 suppliers
$6.00/10g
![(S)-3-PHENYL-2-[(PYRAZIN-2-YLCARBONYL)AMINO] PROPANOIC ACID](/CAS/GIF/114457-94-2.gif)
114457-94-2
336 suppliers
$5.00/250mg
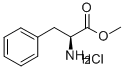
7524-50-7
413 suppliers
$5.00/5g
![(S)-3-PHENYL-2-[(PYRAZIN-2-YLCARBONYL)AMINO] PROPANOIC ACID](/CAS/GIF/114457-94-2.gif)
114457-94-2
336 suppliers
$5.00/250mg