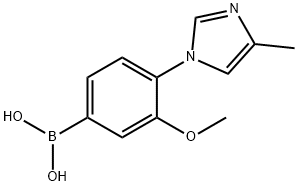
3-methoxy-4-(4-methyl-1H-imidazol-1-yl)phenylboronic acid synthesis
- Product Name:3-methoxy-4-(4-methyl-1H-imidazol-1-yl)phenylboronic acid
- CAS Number:1145786-45-3
- Molecular formula:C11H13BN2O3
- Molecular Weight:232.04
870838-56-5
16 suppliers
$245.43/5g

1145786-45-3
22 suppliers
$155.00/100mg
Yield:1145786-45-3 47%
Reaction Conditions:
with n-butyllithium;Triisopropyl borate in tetrahydrofuran;hexane at -78 - 20;Product distribution / selectivity;
Steps:
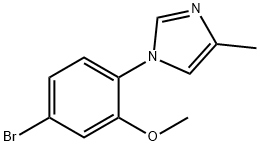
To the solution of 1-(4-bromo-2-methoxyphenyl)-4-methyl-1 /-/-imidazole (51 g, 190.9mmol) and B(i-PrO)3 (53.8g, 286.4mmol) in THF (500ml) was added n-BuLi (381.9mmol, 2.5M in hexane) dropwise at -78 0C. After the addition, the mixture was stirred at -78 0C for 1 hour, allowed to warm to room temperature, and stirred overnight. The reaction was quenched by the addition of saturated NH4CI. The solvent was removed under vacuum. The residue was basified to pH around 12 by mixing with 2M NaOH. After extraction with EtOAc, the aqueous phase was mixed with diluted HCI to give the boronic acid, which was extracted with EtOAc. The combined organic layers were dried over anhydrous Na2SO4. Evaporation of the solvent afforded the title compound as a solid (20.8g, 47%): LC-MS m/z 233 (M+H)+, 1.25 min (ret time)
References:
WO2009/50227,2009,A1 Location in patent:Page/Page column 78

870838-56-5
16 suppliers
$245.43/5g

73183-34-3
587 suppliers
$6.00/5g

1145786-45-3
22 suppliers
$155.00/100mg
![1H-IMidazole, 1-[2-Methoxy-4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)phenyl]-4-Methyl-](/CAS/20150408/GIF/1145786-44-2.gif)
1145786-44-2
5 suppliers
inquiry