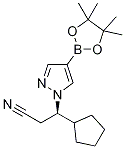
(R)-3-cyclopentyl-3-(4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)propanenitrile synthesis
- Product Name:(R)-3-cyclopentyl-3-(4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)propanenitrile
- CAS Number:1146629-84-6
- Molecular formula:C17H26BN3O2
- Molecular Weight:315.22
![3-Cyclopentyl-3-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl]propanenitrile](/CAS/GIF/1153949-38-2.gif)
1153949-38-2
46 suppliers
inquiry

1146629-84-6
63 suppliers
inquiry
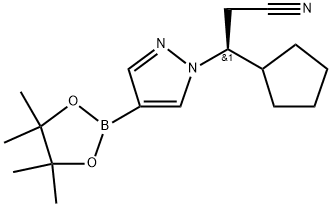
1236033-24-1
1 suppliers
inquiry
Yield: 99.6 % ee , 99.2 % ee
Reaction Conditions:
with Chiralpak IA in hexanes;ethanol at 20;Purification / work up;
Steps:
(R)-3-cyclopentyl-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)propanenitrile ((R)-24) and (S)-3-cyclopentyl-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)propanenitrile ((S)-24).; A solution of racemic 3-cyclopentyl-3-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl]propanenitrile (23, 13.1 g, 41.56 mmol) in a mixture of ethanol and hexanes (8:2 by volume) was injected into preparative HPLC system equipped with a chiral column (20×250 mm) packed with amylose tri(3,5-dimethylphenyl)carbamate immobilized on silicagel (Chiralpak IA) from Chiral Technologies Inc. The chiral column was eluted with mobile phase made by a mixture of ethanol (EtOH) and hexanes in a 1 to 9 volume ratio at a flow rate of 18 mL/min at room temperature. The column elution was monitored by UV at wavelength 220 nm. Under these conditions, a baseline separation of the two enantiomers was achieved and the retention times were 7.0 minutes (Peak 1, the undesired (S)-enantiomer (S)-24) and 8.3 minutes (Peak 2, the desired (R)-enantiomer (R)-24), respectively. Each injection was 0.8 mL of feed solution at a concentration of 100 mg/mL and each run cycle was 14 minutes by using stack injections. Total 164 injections were taken for this separation process. Fractions for Peak 1 (the undesired (S)-enantiomer, (S)-24) and Peak 2 (the desired (R)-enantiomer, (R)-24) were collected separately from each injection, and fractions collected for each peak were concentrated under reduced pressure. The residue from each evaporator was further dried under high vacuum to constant weight to afford (R)-3-cyclopentyl-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)propanenitrile ((R)-24, 6.19 g, 6.55 g theoretical, 94.5% yield) from Peak 2 as off-white solids and (5)-3-cyclopentyl-3-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-pyrazol-1-yl)propanenitrile ((S)-24, 6.08 g, 6.55 g theoretical, 92.8% yield) from Peak 1 as off-white solids.A chiral HPLC method was developed for chiral purity evaluation of both enantiomers of compound 24 ((R)-24 and (S)-24) by using a Chiralpak IA column (4.6×50 mm, 5 μm) purchased from Chiral Technologies, Inc. Two enantiomers ((R)-24 and (S)-24) are separated with a resolution greater than 3.0 by using a mobile phase made from 15% ethanol and 85% hexanes at room temperature with a flow rate of 1 mL/min. The UV detection wavelength is 220 nm. The retention times are 6.4 minutes for (S)-24 and 7.6 minutes for (R)-24, respectively.The quality of each enantiomer separated by preparative chiral HPLC including chemical purity (HPLC area %) and chiral purity (chiral HPLC area %) was analyzed and their structures are confirmed by NMRs and LC/MS. For (R)-24: achiral purity (98.8 area % by HPLC detected at 220 nm); chiral purity (99.8 area % by chiral HPLC; 99.6% ee); 1H NMR (DMSO-d6, 400 MHz) δ ppm 8.07 (d, 1H, J=0.53 Hz), 7.65 (s, 1H), 4.42 (td, 1H, J=19.2, 4.5 Hz), 3.14 (dd, 1H, J=9.39, 17.2 Hz), 3.08 (dd, 1H, J=4.58, 17.2 Hz), 2.31 (m, 1H), 1.75 (m, 1H), 1.62-1.32 (m, 4H), 1.29-1.01 (m, 15H); C17H26BN3O2 (MW, 315.22) LCMS (EI) m/e 316 (M++H). For (S)-24: achiral purity (98.6 area % by HPLC detected at 220 nm); chiral purity (99.6 area % by chiral HPLC; 99.2% ee); 1H NMR (DMSO-d6, 400 MHz) δ ppm 8.07 (d, 1H, J=0.53 Hz), 7.65 (s, 1H), 4.42 (td, 1H, J=19.2, 4.5 Hz), 3.14 (dd, 1H, J=9.39, 17.2 Hz), 3.08 (dd, 1H, J=4.58, 17.2 Hz), 2.31 (m, 1H), 1.75 (m, 1H), 1.62-1.32 (m, 4H), 1.29-1.01 (m, 15H); C17H26BN2O2 (MW, 315.22) LCMS (EI) m/e 316 [M++H].
References:
Zhou, Jiacheng;Liu, Pingli;Lin, Qiyan;Metcalf, Brian W.;Meloni, David;Pan, Yongchun;Xia, Michael;Li, Mei;Yue, Tai-Yuen;Rodgers, James D.;Wang, Haisheng US2010/190981, 2010, A1 Location in patent:Page/Page column 91-92
1146629-83-5
97 suppliers
inquiry

73183-34-3
588 suppliers
$6.00/5g

1146629-84-6
63 suppliers
inquiry
136823-41-1
34 suppliers
$45.00/50mg

1146629-84-6
63 suppliers
inquiry
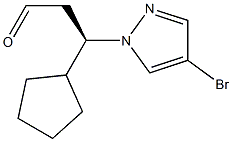
1146629-82-4
16 suppliers
inquiry

1146629-84-6
63 suppliers
inquiry