
8-BROMO-5-HYDROXY-4-(2-HYDROXYETHYL)QUINOLIN-2(1H)-ONE synthesis
- Product Name:8-BROMO-5-HYDROXY-4-(2-HYDROXYETHYL)QUINOLIN-2(1H)-ONE
- CAS Number:1155270-69-1
- Molecular formula:C11H10BrNO3
- Molecular Weight:284.11

1402597-31-2
0 suppliers
inquiry

1155270-69-1
8 suppliers
inquiry
Yield:1155270-69-1 95%
Reaction Conditions:
Stage #1: methyl 2-(8-bromo-5-hydroxy-2-oxo-1,2-dihydroquinolin-4-yl)acetatewith sodium bis(2-methoxyethoxy)aluminium dihydride in tetrahydrofuran;isopropyl alcohol;toluene at 0 - 25; for 4.5 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;isopropyl alcohol;toluene at 40;
Steps:
3
Example 32b 3a2b (20 g, 64 mmol) was charged into a clean and dry reactor followed by addition of THF (140 mL). After the resulting mixture was cooled to 0 °C, Vitride (Red-AI, 47.84 g, 65 wt%, 154 mmol) in toluene was added while maintaining an internal temperature at 0-5 °C. After the batch was agitated at 5-10 °C for 4 hours, IPA (9.24 g, 153.8 mmol) was added while maintaining the temperature below 10 °C. Then the batch was agitated at least for 30 minutes below 25 °C. A solution of HCI in IPA (84.73 g, 5.5 M, 512 mmol) was added into the reactor while maintaining the temperature below 40 °C. After about 160 mL of the solvent was distilled under vacuum below 40 °C, the batch was cooled to 20-25 °C and then aqueous 6M HCI (60 mL) was added while maintaining the temperature below 40 °C. The batch was cooled to 25 °C and agitated for at least 30 minutes. The solid was collected by filtration, washed with 40 mL of IPA and water (1V/1V), 40 mL of water and 40 mL of heptanes. The solid was dried below 60 °C in a vacuum oven to reach KF < 0.5%. Typically, the product 3a was obtained in 90-95% yield with 95 wt%. 1H NMR (400 MHz, DMSO-d6): δ = 10.7 (s, 1 H), 9.68 (s, 1 H), 7.59 (d, 1 H, J = 8.7 Hz), 6.64 (, 1 H, J = 8.7 Hz), 6.27 (s, 1 H), 4.62 (bs, 1 H), 3.69 (t, 2H, J = 6.3 Hz), 3.21 (t, 2H, J = 6.3 Hz).
References:
WO2012/138670,2012,A1 Location in patent:Page/Page column 60

1155270-68-0
6 suppliers
inquiry

1155270-69-1
8 suppliers
inquiry
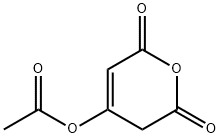
15997-62-3
23 suppliers
$420.00/500mg

1155270-69-1
8 suppliers
inquiry
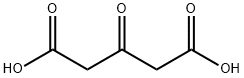
542-05-2
486 suppliers
$6.00/10g

1155270-69-1
8 suppliers
inquiry

1155270-67-9
6 suppliers
inquiry

1155270-69-1
8 suppliers
inquiry