
1-(tetrahydro-2H-pyran)-1H-indazol-6-boronic acid pinacol ester synthesis
- Product Name:1-(tetrahydro-2H-pyran)-1H-indazol-6-boronic acid pinacol ester
- CAS Number:1158680-98-8
- Molecular formula:C18H25BN2O3
- Molecular Weight:328.21
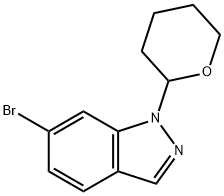
1158680-88-6
60 suppliers
$18.00/250mg

73183-34-3
587 suppliers
$6.00/5g

1158680-98-8
18 suppliers
inquiry
Yield:1158680-98-8 93%
Reaction Conditions:
with (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride;potassium acetate in 1,4-dioxane;Reflux;
Steps:
Description 89I -(tetrahydro-2H-pyran-2-yI)-6-(4,4,5,5-tetramethyl-I ,3,2-dioxaborolan-2-yl)-1 Hindazole (D89)
To a solution of 6-bromo-1 -(tetrahydro-2H-pyran-2-yI)-1 H-indazole (3.5 g, 10.7 mmol),4,4,4’,4’,5,5,5’,5’-octamethyl-2,2’-bi(1 ,3,2-dioxaborolane) (3.25 g, 12.8 mmol) and KOAc(4.82 g, 49.2 mmol) in dioxane (50 mL) was added Pd(dppf)Cl2 (630 mg, 0.86 mmcl). The reaction mixture was refluxed for 2 hours. The reaction mixture was filtered, and the filtrate was concentrated, purified by silica gel chromatography column (petroleum ether/EtOAc = 10/1) to afford the title compound (3.8 g, 93%) as a yellow solid.1H NMR (300 MHz, CDCI3): 58.06 (s, 2H), 7.73(d, J- 8.1Hz, 1H), 7.60 (d, J= 8.1Hz, 1H),5.81 (dd, J 9.6, 2.4 Hz, 1H), 4.10-4.05 (m, 1H), 3.84-3.77 (m, 1H), 2.71-2.58 (m, 1H), 2.21-2.16 (m, 1H), 2.06-2.01 (m, 1H), 1.83-1.69 (m, 3H), 1.40 (s, 12H).
References:
WO2018/137593,2018,A1 Location in patent:Page/Page column 92
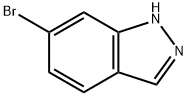
79762-54-2
377 suppliers
$3.00/1g

1158680-98-8
18 suppliers
inquiry
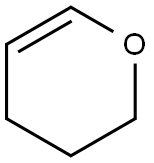
110-87-2
544 suppliers
$10.00/1g

1158680-98-8
18 suppliers
inquiry