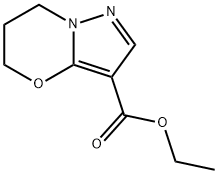
Ethyl 6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-3-carboxylate synthesis
- Product Name:Ethyl 6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-3-carboxylate
- CAS Number:1173003-60-5
- Molecular formula:C9H12N2O3
- Molecular Weight:196.2
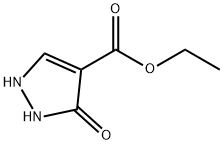
7251-53-8
107 suppliers
$18.00/100mg
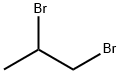
78-75-1
156 suppliers
$22.00/25g
![Ethyl 6,7-dihydro-5H-pyrazolo[5,1-b][1,3]oxazine-3-carboxylate](/CAS/GIF/1173003-60-5.gif)
1173003-60-5
16 suppliers
inquiry
Yield:1173003-60-5 58%
Reaction Conditions:
Stage #1: ethyl 3-oxo-2,3-dihydro-1H-pyrazole-4-carboxylatewith potassium carbonate in N,N-dimethyl-formamide at 130;
Stage #2: 1,2-Dibromopropane in N,N-dimethyl-formamide; for 0.5 h;
Steps:
6,7-Dihydro-5H-pyrazolo[5,l-6] [l,3]oxazine-3-carboxylic acid ethyl ester:[00359] 3-Oxo-2,3-dihydro-lH-pyrazole-4-carboxylic acid ethyl ester (13.2 g, 84.5 mmol), potassium carbonate (50 g, 0.3 mol) and N,N-dimethylformamide (500 mL) were combined in a 250 mL round bottom flask, and the mixture was heated to 130 0C. 1 ,2-Dibromo-propane (10.4 mL, 0.10 mol) was added. After 30 minutes, the mixture was partitioned between DCM and water. The aqueous layer was extracted with DCM and the combined organics were washed with brine, dried (Na2SO4), filtered and concentrated in vacuo. The yellow solid was purified by column chromatography (DCM/MeOη) to afford the ester (9.67 g, 58%) as a solid. 1H NMR (400MHz, DMSO-6) J7.62 (s, IH), 4.41 -4.38 (m, 2H), 4.13 (q, 2H), 4.08 (t, 2H, J= 6.03 Hz), 2.21-2.15 (m, 2H), 1.21 (t, 3H, J= 7.09 Hz).
References:
WO2009/89057,2009,A1 Location in patent:Page/Page column 56