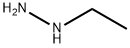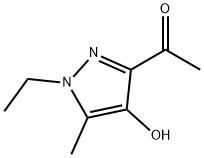
1-(1-Ethyl-4-hydroxy-5-methyl-1H-pyrazol-3-yl)ethanone synthesis
- Product Name:1-(1-Ethyl-4-hydroxy-5-methyl-1H-pyrazol-3-yl)ethanone
- CAS Number:1187732-72-4
- Molecular formula:C8H12N2O2
- Molecular Weight:168.19
6629-60-3
94 suppliers
$52.00/5g
78-98-8
289 suppliers
$10.60/1gm:

1187732-72-4
20 suppliers
inquiry
Yield:1187732-72-4 56%
Reaction Conditions:
with acetic acid in water; for 3 h;Reflux;
Steps:
3
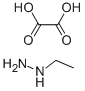
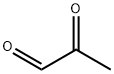
Pyrazolospiroketone Preparation 3; 2'-Ethyl-3'-methyl-2'H-spirorpiperidine-4,5'-Dyranof3.2-clpyrazol1-7'(6'H)-one; An aqueous solution of methyl glyoxal (pyruvaldehyde) (40%, 6.5mL, 40 mmol) was added to a solution of ethylhydrazine oxalate (1 g, 6.7 mmol) and acetic acid (0.57 mL, 10 mmol) in water (11 mL), and the resulting mixture was heated at reflux for 3 hours. The reaction mixture was cooled to room temperature and extracted with EtOAc (3x). The combined organic layers were dried, filtered, and concentrated under reduced pressure. The crude product was purified by flash chromatography (silica gel) eluting with a gradient of
References:
WO2009/144554,2009,A1 Location in patent:Page/Page column 17-18