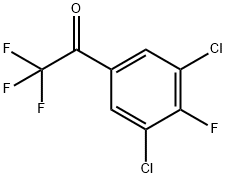
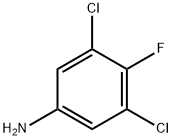
1-(3,5-Dichloro-4-fluorophenyl)-2,2,2-trifluoroethanone synthesis
- Product Name:1-(3,5-Dichloro-4-fluorophenyl)-2,2,2-trifluoroethanone
- CAS Number:1190865-44-1
- Molecular formula:C8H2Cl2F4O
- Molecular Weight:261.0005
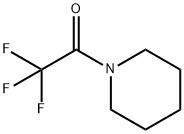
340-07-8
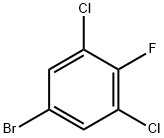
17318-08-0

1190865-44-1
The general procedure for the synthesis of 3,5-dichloro-4-fluorotrifluoroacetylbenzene from 1-trifluoroacetylpiperidine and 3,5-dichloro-4-fluorobromobenzene is as follows: Intermediate 3: Synthesis of 1-(4-chloro-3,5-difluorophenyl)-2,2,2-trifluoroacetophenone 1. 5-bromo-2-fluoro-1,3-dichlorobenzene (7.0 g, 28.7 mmol) was dissolved in THF (50 mL) under argon protection and stirred at room temperature. 2. isopropylmagnesium chloride lithium chloride complex (24.3 mL, 1.3 M solution in THF, 1.1 equiv) was added slowly over 1 min and stirring was continued at room temperature for 30 min. 3. The reaction system was cooled to 0 °C and piperidine trifluoroacetamide (5.6 mL, 1.32 equiv.) was added over about 1 min. 4. The reaction mixture was stirred at room temperature for 2 hours. 5. Upon completion of the reaction, the reaction was quenched with saturated aqueous NH4Cl solution (50 mL). 6. The reaction mixture was extracted with methyl tert-butyl ether (MTBE, 2 x 50 mL). 7. The organic phases were combined and concentrated under reduced pressure to remove the solvent. 8. The crude product was purified by chromatography on a 12 g Redi-Sep column with the eluent being a heptane solution of 0 to 50% ethyl acetate to give 3.5 g of 1-(4-chloro-3,5-difluorophenyl)-2,2,2-trifluoroethanone. Product Characterization: 1H-NMR (400 MHz, CDCl3) δ ppm 8.06 (dd, 2H, J1 = 6.2 Hz, J2 = 0.9 Hz).

340-07-8
79 suppliers
$6.00/1g

17318-08-0
184 suppliers
$6.00/1g

1190865-44-1
140 suppliers
$6.00/1g
Yield:1190865-44-1 3.5 g
Reaction Conditions:
Stage #1:5-bromo-1,3-dichloro-2-fluorobenzene with TurboGrignard in tetrahydrofuran at 20; for 0.516667 h;Inert atmosphere;
Stage #2:N-(trifluoroacetyl)piperidine in tetrahydrofuran at 0 - 20; for 2 h;Inert atmosphere;
Steps:
1 Intermediate 3: 1-(4-chloro-3,5-difluorophenyl)-2,2,2-trifluoroethanone
Intermediate 3: 1-(4-chloro-3,5-difluorophenyl)-2,2,2-trifluoroethanone 5-Bromo-2-fluoro-1 ,3-dichlorobenzene (7.0 g, 28.7 mmol) was stirred at room temperature in THF (50 ml_) under argon and isopropylmagenesium chloride lithium chloride complex (24.3 ml_, 1.3 M in THF, 1.1 eq) was added over 1 min and stirred at RT for 30 min. To this was added piperidine trifluoroacetamide (5.6 ml_, 1.32 eq) over about 1 minute at 0°C and the reaction was stirred at room temperature for 2 h. The reaction was quenched with aqueous saturated NH4CI (50 ml_) and extracted with MTBE (2 x 50 ml_). Solvents were removed under reduced pressure and the crude product was purified using 12 g Redi-Sep column, eluting with 0 to 50% ethyl acetate in heptanes, to yield 3.5 g of 1-(4-chloro-3,5-difluorophenyl)-2,2,2- trifluoroethanone. H-NMR (400 MHz, CDCI3) δ ppm 8.06 (dd, 2H, J1 = 6.2 Hz, J2 = 0.9 Hz).
References:
AVISTA PHARMA SOLUTIONS;SPEAKE, Jason, D.;PERALES, Joe, B.;FAN, Weiming;ROLLAND, Solène, Clarisse WO2016/115315, 2016, A1 Location in patent:Page/Page column 30; 31
431-47-0
292 suppliers
$10.00/5g

17318-08-0
184 suppliers
$6.00/1g

1190865-44-1
140 suppliers
$6.00/1g
2729-34-2
121 suppliers
$5.00/250mg

1190865-44-1
140 suppliers
$6.00/1g

17318-08-0
184 suppliers
$6.00/1g

1190865-44-1
140 suppliers
$6.00/1g
655-32-3
158 suppliers
$9.00/1g

1190865-44-1
140 suppliers
$6.00/1g