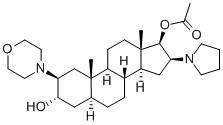
(2b,3a,5a,16b,17b)-17-Acetoxy-3-hydroxy-2-(4-morpholinyl)-16-(1-pyrrolidinyl)androstane synthesis
- Product Name:(2b,3a,5a,16b,17b)-17-Acetoxy-3-hydroxy-2-(4-morpholinyl)-16-(1-pyrrolidinyl)androstane
- CAS Number:119302-24-8
- Molecular formula:C29H48N2O4
- Molecular Weight:488.7
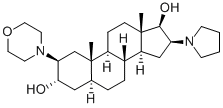
119302-20-4
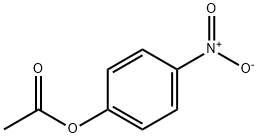
830-03-5

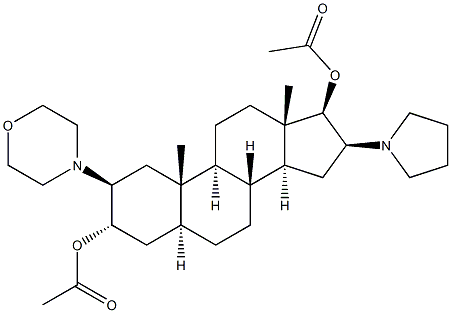
119302-24-8
GENERAL STEPS: To a dry and clean four-necked round-bottomed flask were added (2b,3a,5a,16b,17b)-2-(4-morpholinyl)-16-(1-pyrrolidinyl)androstane-3,17-diol (50.0 g), triethylamine (47.58 mL), 4-nitrophenyl acetate (91.04 g) and dichloromethane (500 mL). The reaction mixture was heated to 80-85 °C and maintained under HPLC monitoring for 7-8 h. HPLC analysis showed that the reaction mixture contained 94-95% 17-monoacetate, 5-6% diacetate and 0.2-0.3% 3,17-diol. Upon completion of the reaction, the reaction mixture was quenched with water, followed by post-processing to isolate (2b,3a,5a,16b,17b)-17-acetoxy-3-hydroxy-2-(4-morpholinyl)-16-(1-pyrrolidinyl)androstane. The yield was 51.3 g (94.1% yield) and the HPLC purity was as follows: greater than 98% for 17-monoacetate and no more than 2.0% for diacetate. Further purification by acetonitrile crystallization gave the target product with purity greater than 99.5%.

119302-20-4
257 suppliers
$34.00/250mg

75-36-5
589 suppliers
$17.92/100G

119302-24-8
186 suppliers
$59.00/25mg
119302-22-6
11 suppliers
inquiry
Yield:119302-24-8 94.1 %Chromat. ,119302-22-6 2.1 %Chromat.
Reaction Conditions:
in dichloromethane at 23; for 24 h;Product distribution / selectivity;
Steps:
1
Acetyl chloride (23.0 ml, 0.3234 mole) was added to a solution of Compound II (107 grams, 0.2395 mole) in 2 liters of dichloromethane. The reaction mixture was set-aside for 24 hours at room temperature (about 23° C.). HPLC analysis of a reaction sample showed that the mixture contained 35.1% of Compound I, 0.3% of Compound II and 55.5% of Compound III. Aqueous HCl solution (10.5%, 305 ml) was added thereafter and the mixture was heated to reflux for 4 hours. After cooling to 2° C., the mixture was neutralized to pH 7.2 by adding 5 liters of 5% sodium bicarbonate solution, and the aqueous phase was removed. HPLC analysis of the organic phase showed a composition of 86.3% of the desired Compound I, 1.9% of Compound II and 8.8% of Compound III. The organic phase was washed with two portions of 500 ml of water, dried with Na2SO4 and the dichloromethane was removed by evaporation. The residue was obtained as yellow crystals (98.4 grams, 84% yield). HPLC analysis of the residue showed it contained 94.1% of the desired Compound I, 1.6% of Compound II and 2.1% of Compound III.
References:
Adar, Eliezer;Sondack, David;Friedman, Oded;Manascu, Iosef;Fizitzki, Tamir;Freger, Boris;Arad, Oded;Weisman, Alexander;Kaspi, Joseph US2005/159398, 2005, A1 Location in patent:Page/Page column 8

119302-20-4
257 suppliers
$34.00/250mg

830-03-5
194 suppliers
$10.00/5g

119302-24-8
186 suppliers
$59.00/25mg

119302-20-4
257 suppliers
$34.00/250mg

64-19-7
1628 suppliers
$10.00/25ML

119302-24-8
186 suppliers
$59.00/25mg

119302-20-4
257 suppliers
$34.00/250mg

108-24-7
0 suppliers
$14.00/250ML

119302-24-8
186 suppliers
$59.00/25mg

119302-22-6
11 suppliers
inquiry

119302-22-6
11 suppliers
inquiry

119302-24-8
186 suppliers
$59.00/25mg