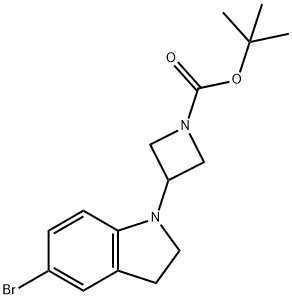
3-(5-broMo-2,3-dihydro-indol-1-yl)-azetidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:3-(5-broMo-2,3-dihydro-indol-1-yl)-azetidine-1-carboxylic acid tert-butyl ester
- CAS Number:1194732-22-3
- Molecular formula:C16H21BrN2O2
- Molecular Weight:353.25
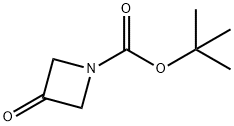
398489-26-4
442 suppliers
$9.00/5g
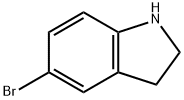
22190-33-6
247 suppliers
$9.00/1g

1194732-22-3
1 suppliers
inquiry
Yield:1194732-22-3 93%
Reaction Conditions:
with sodium tris(acetoxy)borohydride;acetic acid in dichloromethane; for 3 h;Inert atmosphere;
Steps:
3-(5-Bromo-2,3-dihydro-indol-1-yl)-azetidine-1-carboxylic acid tert-butyl ester
To a solution of 5-bromoindoline (30.0 g, 151 mmol) in DCM (150 mL) was added tert-butyl-3-oxoazetidine-1-carboxylate (28.5 g, 167 mmol), acetic acid (1.00 mL, 17.6 mmol) and sodium triacetoxyborohydride (68.5 g, 323 mmol). The reaction mixture was stirred for 3 h and diluted with EtOAc. The organic layer was washed with saturated citric acid, saturated NaHCO3, and brine, and driedover MgSO4. Removal of solvent gave crude product which was used without further purification (50g ascrude product, ~94% pure by LC/MS).
References:
Lu, Kai;Jiang, Yu;Chen, Bin;Eldemenky, Eman M.;Ma, Gil;Packiarajan, Mathivanan;Chandrasena, Gamini;White, Andrew D.;Jones, Kenneth A.;Li, Boshan;Hong, Sang-Phyo [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 18,p. 5310 - 5314] Location in patent:supporting information; experimental part