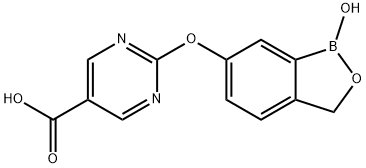
2-((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)pyrimidine-5-carboxylic acid synthesis
- Product Name:2-((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)pyrimidine-5-carboxylic acid
- CAS Number:1196473-62-7
- Molecular formula:C12H9BN2O5
- Molecular Weight:272.02
![Ethyl 2-((1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)pyrimidine-5-carboxylate](/CAS/20180702/GIF/1196473-61-6.gif)
1196473-61-6
4 suppliers
inquiry
![2-((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)pyrimidine-5-carboxylic acid](/CAS/20180629/GIF/1196473-62-7.gif)
1196473-62-7
3 suppliers
inquiry
Yield:1196473-62-7 21%
Reaction Conditions:
with lithium hydroxide in methanol;water at 0 - 20;
Steps:
Experimental procedure for the preparation of compound 24
To a solution of 23 (0.5 g, 1.75 mmol) in methanol (20 mL) was added aqueous LiOH (0.419 g in 15 mL of water, 17.5 mmol) at 0 °C. The resulting mixture was stirred at room temperature for 1.5 h. After removed most of the methanol, the reaction mixture was cooled to 0 °C and acidified to pH 2 using diluted hydrochloric acid. The white precipitate was collected, washed with water and dried to give the crude product which was purified by chromatography on silica gel (hexane/THF/AcOH = 2:1:trace) to give 0.102 g (21%) of 24; mp 195-196 °C.
References:
Xia, Yi;Cao, Kathy;Zhou, Yasheen;Alley;Rock, Fernando;Mohan, Manisha;Meewan, Maliwan;Baker, Stephen J.;Lux, Sarah;Ding, Charles Z.;Jia, Guofeng;Kully, Maureen;Plattner, Jacob J. [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 8,p. 2533 - 2536] Location in patent:experimental part
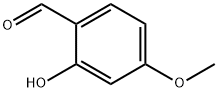
673-22-3
496 suppliers
$5.00/1g
![2-((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)pyrimidine-5-carboxylic acid](/CAS/20180629/GIF/1196473-62-7.gif)
1196473-62-7
3 suppliers
inquiry
![benzo[c][1,2]oxaborole-1,6(3H)-diol](/CAS/20150408/GIF/1196473-37-6.gif)
1196473-37-6
53 suppliers
$99.00/100mg
![2-((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)pyrimidine-5-carboxylic acid](/CAS/20180629/GIF/1196473-62-7.gif)
1196473-62-7
3 suppliers
inquiry

1196474-59-5
11 suppliers
inquiry
![2-((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)pyrimidine-5-carboxylic acid](/CAS/20180629/GIF/1196473-62-7.gif)
1196473-62-7
3 suppliers
inquiry
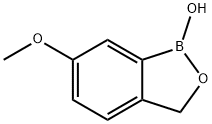
947163-26-0
17 suppliers
inquiry
![2-((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)pyrimidine-5-carboxylic acid](/CAS/20180629/GIF/1196473-62-7.gif)
1196473-62-7
3 suppliers
inquiry