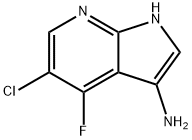
1H-Pyrrolo[2,3-b]pyridin-3-aMine, 5-chloro-4-fluoro- synthesis
- Product Name:1H-Pyrrolo[2,3-b]pyridin-3-aMine, 5-chloro-4-fluoro-
- CAS Number:1196507-37-5
- Molecular formula:C7H5ClFN3
- Molecular Weight:185.59
![1H-Pyrrolo[2,3-b]pyridine, 5-chloro-4-fluoro-3-nitro-](/CAS/GIF/1196507-34-2.gif)
1196507-34-2
9 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridin-3-aMine, 5-chloro-4-fluoro-](/CAS/GIF/1196507-37-5.gif)
1196507-37-5
3 suppliers
inquiry
Yield:1196507-37-5 77%
Reaction Conditions:
Stage #1: 5-chloro-4-fluoro-3-nitro-1H-pyrrolo[2,3-b]pyridinewith hydrogenchloride;tin(ll) chloride at 20; for 0.5 h;
Stage #2: with sodium hydroxide in water; pH=8;
Steps:
L
[00296] 5-Chloro-4-fluoro-3-nitro-lH-pyrrolo[2,3-b]pyridine (1.20 g, 5.57 mmol) was placed in 6M HCl (30 mL). SnCl2 (5.28 g, 27.8 mmol) was then added, and the reaction was stirred for 30 minutes at room temperature. The reaction was then poured into a mixture of IM NaOH and ice. The resulting suspension was then raised to a pH of 8 and then extracted with 3 : 1 DCM:i-PrOH. The combined organic fractions were dried, filtered, and concentrated to give the crude product, which was triturated with 10:1 hexanes:DCM to give the product 5-chloro-4- fluoro-lH-pyrrolo[2,3-b]pyridin-3-amine (0.8 g, 77% yield).
References:
WO2009/140320,2009,A1 Location in patent:Page/Page column 85
![1H-Pyrrolo[2,3-b]pyridine, 4-fluoro-1-[tris(1-methylethyl)silyl]-](/CAS/GIF/640735-25-7.gif)
640735-25-7
42 suppliers
$134.00/250mg
![1H-Pyrrolo[2,3-b]pyridin-3-aMine, 5-chloro-4-fluoro-](/CAS/GIF/1196507-37-5.gif)
1196507-37-5
3 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridine, 4-bromo-1-[tris(1-methylethyl)silyl]-](/CAS/GIF/640735-24-6.gif)
640735-24-6
31 suppliers
$104.00/500mg
![1H-Pyrrolo[2,3-b]pyridin-3-aMine, 5-chloro-4-fluoro-](/CAS/GIF/1196507-37-5.gif)
1196507-37-5
3 suppliers
inquiry
![5-chloro-4-fluoro-1H-pyrrolo[2,3-b]pyridine](/CAS/GIF/1190317-94-2.gif)
1190317-94-2
34 suppliers
$240.00/50mg
![1H-Pyrrolo[2,3-b]pyridin-3-aMine, 5-chloro-4-fluoro-](/CAS/GIF/1196507-37-5.gif)
1196507-37-5
3 suppliers
inquiry