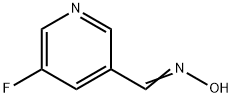
(E)-N-[(5-fluoropyridin-3-yl)Methylidene]hydroxylaMine synthesis
- Product Name:(E)-N-[(5-fluoropyridin-3-yl)Methylidene]hydroxylaMine
- CAS Number:1198353-49-9
- Molecular formula:C6H5FN2O
- Molecular Weight:140.12
39891-04-8
142 suppliers
$20.00/1g
![(E)-N-[(5-fluoropyridin-3-yl)Methylidene]hydroxylaMine](/CAS/20150408/GIF/1198353-49-9.gif)
1198353-49-9
14 suppliers
$230.00/250mg
Yield:-
Reaction Conditions:
with hydroxylamine hydrochloride;sodium acetate in ethanol at 20; for 12 h;
Steps:
28.A. 5-Fluoronicotinaldehyde oxime
The titled compound was prepared according to Method OB using 5-fluoronicotinaldehyde (Aldrich). 1H NMR (300 MHz, MeOH-d4) δ 7.85 (dt, J=9.5, 2.2 Hz, 1H), 8.16 (s, 1H), 8.43 (d, J=2.7 Hz, 1H), 8.57 (s, 1H) ppm; MS (DCI/NH3) m/z 141 (M+H)+.; Method OB: To a solution of aryl aldehyde or heteroaryl aldehyde (10.0 mmol) in EtOH (10 mL) was added hydroxylamine hydrochloride (Aldrich, 0.96 g, 15.0 mmol) and sodium acetate (Aldrich, 1.14 g, 15.0. mmol). The mixture was stirred at ambient temperature for 12 hours. Then the reaction mixture was diluted with EtOAc (100 mL) and washed with water (2×10 mL) and brine (2×10 mL). The organic solution was dried over anhydrous Na2SO4 for 1 hour. The drying agent was removed by filtration, and the organic solution was concentrated to give the title product.
References:
US2009/306096,2009,A1 Location in patent:Page/Page column 9; 13