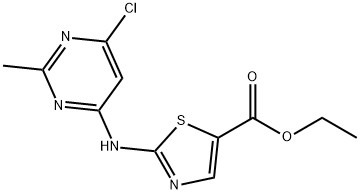
2-(6-Chloro-2-methylpyrimidin-4-ylamino)thiazole-5-carboxylic acid ethyl ester synthesis
- Product Name:2-(6-Chloro-2-methylpyrimidin-4-ylamino)thiazole-5-carboxylic acid ethyl ester
- CAS Number:1202357-66-1
- Molecular formula:C11H11ClN4O2S
- Molecular Weight:298.75
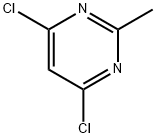
1780-26-3
478 suppliers
$10.00/10g
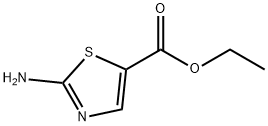
32955-21-8
372 suppliers
$7.00/1g
![(S)-1'-(tert-butyl)-2'-oxo-1',2',5,7-tetrahydrospiro[cyclopenta[b]pyridine-6,3'-pyrrolo[2,3-b]pyridine]-3-carboxylic acid](/CAS/20200401/GIF/1455358-16-3.gif)
1455358-16-3
19 suppliers
inquiry
Yield:1455358-16-3 98%
Reaction Conditions:
with sodium hydride in N,N-dimethyl-formamide at 0 - 20; for 72 h;Inert atmosphere;
Steps:
I I. Ethyl 2-((6-chloro-2-methylpyrimidin-4-yl)amino)thiazole-5-carboxylate:
To a solution of 4,6-dichloro-2-methylpyrimidine (10 g, 61.4 mmol, 1.0 eq.) and ethyl 2-aminothiazole-5-carboxylate (10.6 g, 61.4 mmol, 1.0 eq.) in DMF (210 ml) at 0°C under inert atmosphere was added sodium hydride (5.40 g, 135 mmol, 2.0 eq.) in portions and the reaction mixture was slowly warmed to room temperature and stirred for 3d.
Excess of the NaH was quenched by addition of saturated solution of ammonium chloride and the reaction mixture was poured on water (3000 ml) and stirred for 1h at room temperature.
Obtained precipitate was filtered off and air dried to get ethyl 2-((6-chloro-2-methylpyrimidin-4-yl)amino)thiazole-5-carboxylate (18 g, 60.0 mmol, 98 % yield) as a beige solid.
1H NMR (400 MHz, CDCl3) δ 1.34 - 1.51 (t, 3H), 2.75 (s, 3H), 4.41 (q, J = 7.1 Hz, 2H), 6.73 (s, 1H), 8.14 (s, 1H). LCMS: m/z = 297.1 [M-H]-.
References:
EP3643713,2020,A1 Location in patent:Paragraph 0425-0427