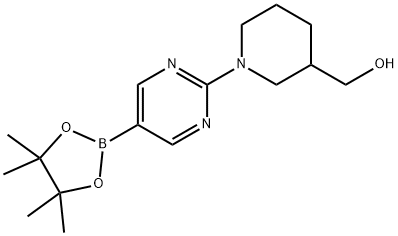
{1-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidin-2-yl]piperidin-3-yl}methanol synthesis
- Product Name:{1-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidin-2-yl]piperidin-3-yl}methanol
- CAS Number:1202805-21-7
- Molecular formula:C16H26BN3O3
- Molecular Weight:319.21
![[1-(5-Bromopyrimidin-2-yl)piperidin-3-yl]methanol](/CAS/GIF/1189973-29-2.gif)
1189973-29-2
29 suppliers
$10.00/250mg

73183-34-3
587 suppliers
$6.00/5g
![{1-[5-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyrimidin-2-yl]piperidin-3-yl}methanol](/CAS/20210111/GIF/1202805-21-7.gif)
1202805-21-7
7 suppliers
inquiry
Yield:-
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in 1,4-dioxane at 25 - 90;Inert atmosphere;
Steps:
A.2
Step 2: Synthesis of {l-[5-(4,4,5,5-tetramethyl-l,3,2-dioxaborolan-2-yl)pyrimidin-2- yl]piperidin-3-yl}methanol :; To a solution of [l-(5-bromopyrimidin-2-yl)piperidin-3-yl]methanol (0.5 g, 1.83 mmol) in 1,4 dioxane (25 mL) were added bispinacolatodiborane (0.46 g, 3.3 mmol), potassium acetate ( 0.53 g, 5.4 mmol) and Pd (dppf)Cl2 (0.14 g, 0.18 mmol) under argon at room temperature (~25 0C). The mixture was heated at 80-90 0C for about 6 hours. It was cooled to room temperature (-25 0C), concentrated under reduced pressure and then purified through filtering column using sintered funnel full of 230-400 mesh size silica gel. It was eluted with 50% ethyl acetate in hexane and concentrated to afford title compound (0.4 g). MS m/e 289.93 (MH+).
References:
WO2009/156966,2009,A1 Location in patent:Page/Page column 35