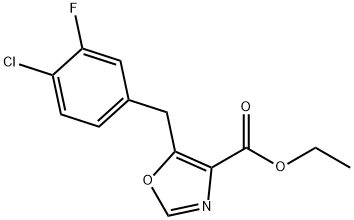
ethyl 5-(4-chloro-3-fluorobenzyl)oxazole-4-carboxylate synthesis
- Product Name:ethyl 5-(4-chloro-3-fluorobenzyl)oxazole-4-carboxylate
- CAS Number:1202897-24-2
- Molecular formula:C13H11ClFNO3
- Molecular Weight:283.68
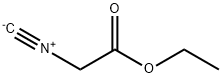
2999-46-4
216 suppliers
$13.43/1gm:
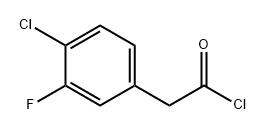
1202897-23-1
1 suppliers
inquiry

1202897-24-2
10 suppliers
inquiry
Yield:1202897-24-2 67.7%
Reaction Conditions:
Stage #1: Ethyl isocyanoacetatewith potassium tert-butylate in tetrahydrofuran at 5;
Stage #2: 2-(4-chloro-3-fluorophenyl)acetyl chloride in tetrahydrofuran at 20;
Steps:
25
To a solution of potassium tert-butoxide (3.5g, 31.25mmol) in THF (50 ml) was added ethyl isocyanoacetate (3.5g, 31.25mmol) dropwise at 5°C. After stirring for 45 minutes, the product of example 24 (3.2g, 15.5mmol) was added dropwise. Then the mixture was stirred overnight at room temperature. The reaction mixture was filtered and the filtrate was concentrated. The residue was purified by column chromatography (PE/EA=5/1) to give the titled compound (2.5g, 67.7yield)1H NMR (300 MHz, DMSO-d6) 1.29 (t, J= 7.1 Hz, 3H), 4.30 (q, J= 7.1 Hz, 2H), 4.41 (s, 2H), 7.11 (ddd, J= 0.6, 2.1, 8.3 Hz, IH), 7.34 (dd, J= 2.0, 10.4 Hz, IH), 7.54 (t, J= 8.1 Hz, IH), 8.40 (s, IH).MS (ESI+) m/z 306 (M+23)
References:
WO2010/31,2010,A1 Location in patent:Page/Page column 56